Sample Paper
PART-II: CHEMISTRY
SECTION-1 : (Maximum Marks: 12)
- This section contains four (04) questions.
- Each question has FOUR options (A), (B), (C), and (D). Only one of these four options is the correct answer.
- For each question, choose the option corresponding to the correct answer.
- Answers to each question will be evaluated according to the following marking scheme:
Full Marks: “+3” If ONLY the correct option is chosen;
Zero Marks: “0” If none of the options is chosen (i.e. the question is unanswered);
Negative Marks: “−1” In all other cases.
Q1. The major product formed in the following reaction is
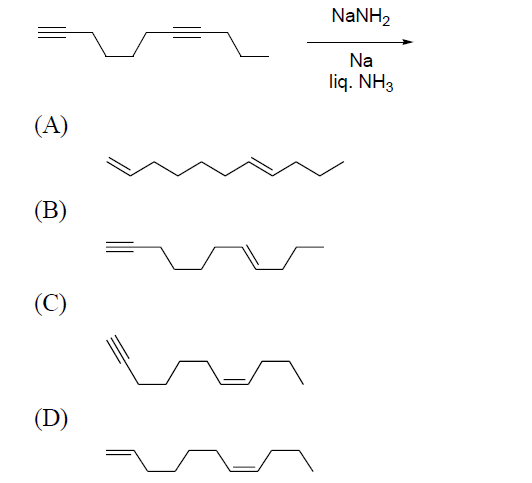
Answer: B
Q2. Among the following, the conformation that corresponds to the most stable conformation of meso–butane–2,3–diol is –

Answer: B
Q3. For the given close packed structure of a salt made of cation \(\mathbf{X}\) and anion \(\mathbf{Y}\) shown below (ions of only one face are shown for clarity), the packing fraction is approximately (packing fraction \(=\frac{\text { packing efficiency }}{100}\) )
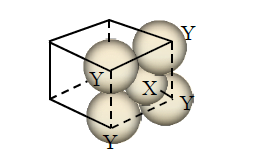
(A) 0.74
(B) 0.63
(C) 0.52
(D) 0.48
Answer: B
Solution:
Packing fraction \((f)=\frac{3 \times \frac{4}{3} \pi r_{+}^{3}+1 \times \frac{4}{3} \pi r_{-}^{3}}{a^{3}}\)
\(
=\frac{1 \times \frac{4}{3} \pi\left[3\left(\frac{r_{+}}{r_{-}}\right)^{3}+1\right]}{\left(\frac{a}{r_{-}}\right)^{3}}
\)
Now \(2 r_{-}=a\)
\(
\therefore \frac{a}{r_{-}}=2
\)
Also, \(\frac{r_{+}}{r_{-}}=0.414\)
So, \(f=\frac{1 \times \frac{4}{3} \times 3.14\left[3 \times(0.414)^{3}+1\right]}{(2)^{3}}\) \(=0.634 \approx 0.63\)
Q4. The calculated spin only magnetic moments of \(\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\) and \(\left[\mathrm{CuF}_{6}\right]^{3-}\) in \(\mathrm{BM}\), respectively, are (Atomic numbers of \(\mathrm{Cr}\) and \(\mathrm{Cu}\) are 24 and 29 , respectively)
(A) 3.87 and 2.84
(B) 4.90 and 1.73
(C) 3.87 and 1.73
(D) 4.90 and 2.84
Answer: A
Solution:
\(\left[ Cr \left( NH _{3}\right)_{6}\right]^{3+}\) has \(d ^{3}\) configuration, so as per CFT, \(N =3\) and \(\mu=\sqrt{3(3+2)}=3.87 BM\) \(\left[ CuF _{6}\right]^{3-}\), has \(d ^{8}\) configuration and weak field ligand. So \(N =2\) and \(\mu=\sqrt{2(2+2)}=2.84 BM\)
SECTION-2 : (Maximum Marks : 12)
- This section contains three (03) question stems.
- There are two (02) questions corresponding to each question stem.
- The answer to each question is a numerical value.
- For each question, enter the correct numerical value corresponding to the answer in the designated place using the mouse and the on-screen virtual numeric keypad.
- If the numerical value has more than two decimal places, truncate/round off the value to two decimal places.
- Answers to each question will be evaluated according to the following marking scheme:
Full Marks: “+2” If only the correct numerical value is entered at the designated place;
Zero Marks: “0” In all other cases.
Question Stem( Question Number 5 & 6)
For the following reaction scheme, percentage yields are given along the arrow:
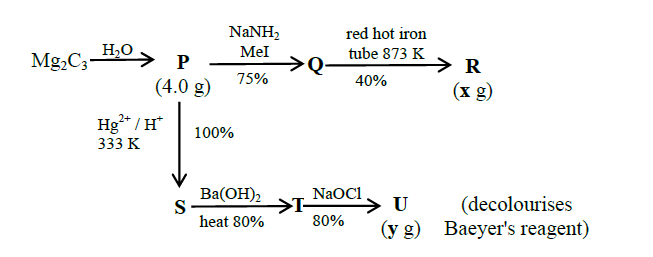
\(\mathbf{x} \mathrm{g}\) and \(\mathbf{y} \mathrm{g}\) are mass of \(\mathbf{R}\) and \(\mathbf{U}\), respectively.
(Use : Molar mass (in \(\mathrm{g} \mathrm{mol}{ }^{-1}\) ) of \(\mathrm{H}, \mathrm{C}\) and \(\mathrm{O}\) as 1,12 and 16 , respectively).
Q5. The value of \(\mathbf{x}\) is _________.
Answer: 1.62
Q6. The value of y is______.
Answer: 3.2
Question Stem for Question Nos. 7 and 8
For the reaction \(\mathbf{X}(\mathrm{s}) \rightleftharpoons \mathbf{Y}(\mathrm{s})+\mathbf{Z}(\mathrm{g})\), the plot of \(\ln \frac{\mathrm{p}_{z}}{\mathrm{p}^{\theta}}\) versus \(\frac{10^{4}}{T}\) is given below (in solid line), where \(\mathrm{p}_{\mathrm{z}}\) is the pressure (in bar) of the gas \(\mathbf{Z}\) at temperature \(T\) and \(\mathrm{P}^{\theta}=1\) bar. (Given, \(\frac{\mathrm{d}(\ln \mathrm{K})}{\mathrm{d}\left(\frac{1}{T}\right)}=-\frac{\Delta H^{\theta}}{\mathrm{R}}\), where the equilibrium constant, \(\mathrm{K}=\frac{\mathrm{p}_{\mathrm{z}}}{\mathrm{p}^{\theta}}\) and the gas constant, \(\mathrm{R}=8.314\) \(\mathrm{J} \mathrm{K}^{-1} \mathrm{~mol}^{-1}\) )
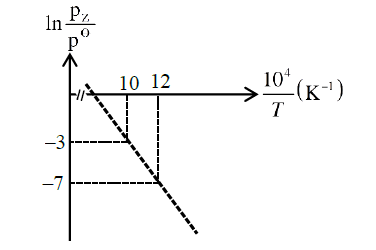
Q7. The value of standard enthalpy, \(\Delta \mathrm{H}^{\theta}\) (in \(\mathrm{kJ} \mathrm{mol}^{-1}\) ) for the reaction is ________.
Answer: 166.28
Solution:
\(\begin{aligned}
&\Delta \mathrm{G}^{\circ}=-\mathrm{RT} \ln \left(\frac{\mathrm{P}}{1}\right)=\Delta \mathrm{H}^{\circ}-\mathrm{T} \Delta \mathrm{S}^{\circ} \\
&\ln \left(\frac{\mathrm{P}}{1}\right)=-\frac{\Delta \mathrm{H}^{\circ}}{\mathrm{RT}}+\frac{\Delta \mathrm{S}^{\circ}}{\mathrm{R}} \\
&\text { Slope }=-\frac{\Delta \mathrm{H}^{\circ}}{\mathrm{R}}=10^{4} \times\left(-\frac{4}{2}\right) \\
&\Rightarrow \Delta \mathrm{H}^{\circ}=2 \times 10^{4} \times \mathrm{R} \\
&=166.28 \mathrm{~kJ} / \mathrm{mole}
\end{aligned}\)\\
Q8. The value of \(\Delta \mathrm{S}^{\ominus}\) (in \(\mathrm{J} \mathrm{K}^{-1} \mathrm{~mol}^{-1}\) ) for the given reaction, at \(1000 \mathrm{~K}\) is __________.
Answer: 141.34
Solution:
From the plot when, \(\frac{10^{4}}{\mathrm{~T}}=10 \Rightarrow \mathrm{T}=1000 \mathrm{~K}\)
\(\ln \left(\frac{\mathrm{P}_{2}}{1}\right)=-3\)
Substituting in the equation:
\(
\ln \left(\frac{\mathrm{P}_{2}}{1}\right)=-\frac{\Delta \mathrm{H}^{\circ}}{\mathrm{RT}}+\frac{\Delta \mathrm{S}^{\circ}}{\mathrm{R}}
\)
We get,
\(
\begin{aligned}
&-3=-\frac{2 \times 10^{4} \times \mathrm{R}}{\mathrm{R} \times 1000}+\frac{\Delta \mathrm{S}^{\circ}}{\mathrm{R}} \\
&\Rightarrow \Delta \mathrm{S}^{\circ}=17 \mathrm{R} \\
&\Rightarrow \Delta \mathrm{S}^{\circ}=17 \times 8.314 \mathrm{~J} / \mathrm{K}-\mathrm{mol} \\
&\Rightarrow \Delta \mathrm{S}^{\circ}=141.34 \mathrm{~J} / \mathrm{K}-\mathrm{mol}
\end{aligned}
\)
Question Stem for Question Nos. 9 and 10
The boiling point of water in a 0.1 molal silver nitrate solution (solution \(\mathbf{A}\) ) is \(\mathbf{x}^{\circ} \mathrm{C}\). To this solution \(\mathbf{A}\), an equal volume of 0.1 molal aqueous barium chloride solution is added to make a new solution \(\mathbf{B}\). The difference in the boiling points of water in the two solutions \(\mathbf{A}\) and \(\mathbf{B}\) is \(\mathbf{y} \times 10^{-2}{ }^{\circ} \mathrm{C}\).
(Assume : Densities of the solutions \(\mathbf{A}\) and \(\mathbf{B}\) are the same as that of water and the soluble salts dissociate completely.)
Use: Molal elevation constant (Ebullioscopic Constant), \(\mathrm{K}_{\mathrm{b}}=0.5 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}\); Boiling point of pure water as \(100^{\circ} \mathrm{C}\).)
Q9. The value of x is _______.
Answer: 100.10
Solution:
\(
\begin{aligned}
&\mathrm{AgNO}_{3}(\mathrm{aq}) \longrightarrow \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{NO}_{3}^{-}(\mathrm{aq}) \\
& 0.1 \mathrm{~m} 0.1 \mathrm{~m}\\
&\Delta \mathrm{T}_{\mathrm{b}}=0.2 \times 0.5 \\
&\quad=0.1^{\circ} \mathrm{C}=0.1 \mathrm{~K}
\end{aligned}
\)
Boiling point of solution \(=100.1^{\circ} \mathrm{C}\)
Q10. The value of \(|\mathbf{y}|\) is _________.
Answer: 2.5
SECTION-3 : (Maximum Marks: 24)
- This section contains six (06) questions.
- Each question has FOUR options (A), (B), (C), and (D). one or more than one of these four option(s) is (are) correct answer(s).
- For each question, choose the option(s) corresponding to (all) the correct answer(s).
- Answer to each question will be evaluated according to the following marking scheme:
Full Marks: “+4” If only (all) the correct option(s) is(are) chosen;
Partial Marks: “+3” If all the four options are correct but ONLY three options are chosen;
Partial Marks: “+2” If three or more options are correct but ONLY two options are chosen, both of which are correct;
Partial Marks: “+1” If two or more options are correct but ONLY one option is chosen and it is a correct option;
Zero Marks: “0” If unanswered;
Negative Marks: “−2” In all other cases. - For example, in a question, if (A), (B), and (D) are the only three options corresponding to correct answers, then
choosing only (A), (B), and (D) will get +4 marks;
choosing only (A) and (B) will get +2 marks;
choosing only (A) and (D) will get +2 marks;
choosing only (B) and (D) will get +2 marks;
choosing only (A) will get +1 mark;
choosing only (B) will get +1 mark;
choosing only (D) will get +1 mark;
choosing no option(s) (i.e. the question is unanswered) will get 0 marks and choosing any other option(s) will get −2 marks.
Q11. Given
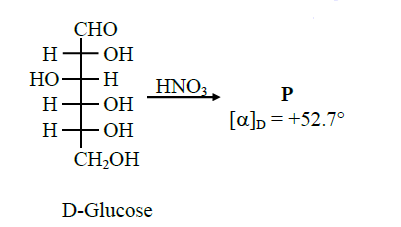
The compound(s), which on reaction with \(\mathrm{HNO}_{3}\) will give the product having degree of rotation,
\(
[\alpha]_{\mathrm{D}}=-52.7^{\circ} \text { is (are) }
\)
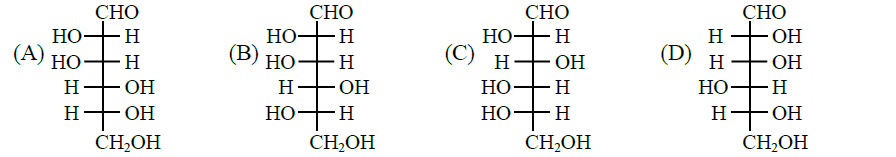
Answer: C & D
Q12. The reaction of \(\mathbf{Q}\) with PhSNa yields an organic compound (major product) that gives positive Carius test on treatment with \(\mathrm{Na}_{2} \mathrm{O}_{2}\) followed by addition of \(\mathrm{BaCl}_{2}\). The correct option(s) for \(\mathbf{Q}\) is (are).

Answer: A & D
Solution:
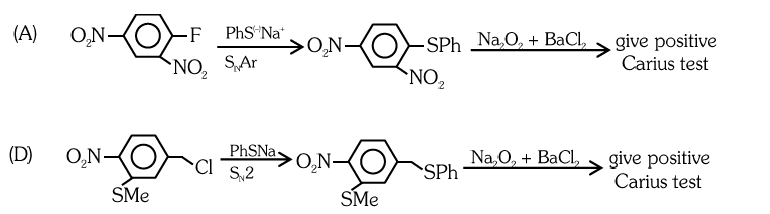
Q13. The correct statement(s) related to colloids is(are)
(A) The process of precipitating colloidal sol by an electrolyte is called peptization.
(B) Colloidal solution freezes at a higher temperature than the true solution at the same concentration.
(C) Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature
(D) Micelles are macromolecular colloids.
Answer: B & C
Q14. An ideal gas undergoes a reversible isothermal expansion from state-I to state-II followed by a reversible adiabatic expansion from state-II to state-III. The correct plot(s) representing the changes from state \(\mathbf{I}\) to state-III is(are)
( \(p\) : pressure, \(V\) : volume, \(T\) : temperature, \(H\) : enthalpy, \(S\) : entropy)
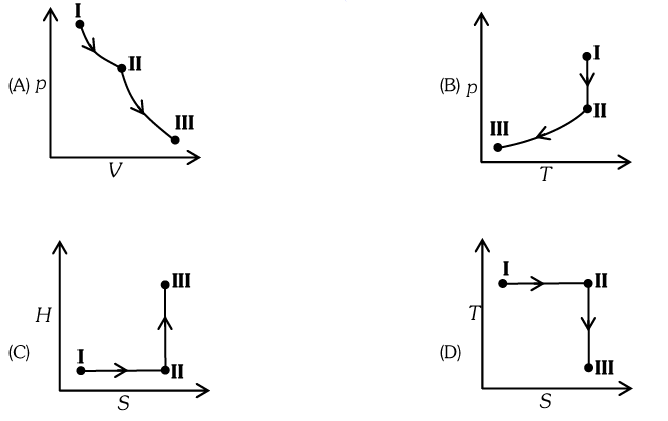
Answer: A, B & D
Q15. The correct statement(s) related to the metal extraction processes is(are)
(A) A mixture of \(\mathrm{PbS}\) and \(\mathrm{PbO}\) undergoes self-reduction to produce \(\mathrm{Pb}\) and \(\mathrm{SO}_{2}\).
(B) In the extraction process of copper from copper pyrites, silica is added to produce copper silicate.
(C) Partial oxidation of sulphide ore of copper by roasting, followed by self-reduction produces blister copper.
(D) In cyanide process, zinc powder is utilized to precipitate gold from \(\mathrm{Na}\left[\mathrm{Au}(\mathrm{CN})_{2}\right]\)
Answer: A, C & D
Q16. A mixture of two salts is used to prepare a solution $\mathbf{S}$, which gives the following results:
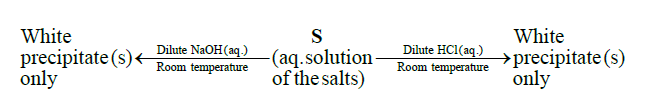
The correct option(s) for the salt mixture is(are)
(A) \(\mathrm{Pb}\left(\mathrm{NO}_{3}\right)_{2}\) and \(\mathrm{Zn}\left(\mathrm{NO}_{3}\right)_{2}\)
(B) \(\mathrm{Pb}\left(\mathrm{NO}_{3}\right)_{2}\) and \(\mathrm{Bi}\left(\mathrm{NO}_{3}\right)_{3}\)
(C) \(\mathrm{AgNO}_{3}\) and \(\mathrm{Bi}\left(\mathrm{NO}_{3}\right)_{3}\)
(D) \(\mathrm{Pb}\left(\mathrm{NO}_{3}\right)_{2}\) and \(\mathrm{Hg}\left(\mathrm{NO}_{3}\right)_{2}\)
Answer: A & B
SECTION-4 : (Maximum Marks: 12)
- This section contains three (03) questions.
- The answer to each question is a non-negative integer.
- For each question, enter the correct integer corresponding to the answer using the mouse and the on-screen virtual numeric keypad in the place designated to enter the answer.
- Answer to each question will be evaluated according to the following marking scheme:
Full Marks: +4 If ONLY the correct integer is entered;
Zero Marks: 0 In all other cases.
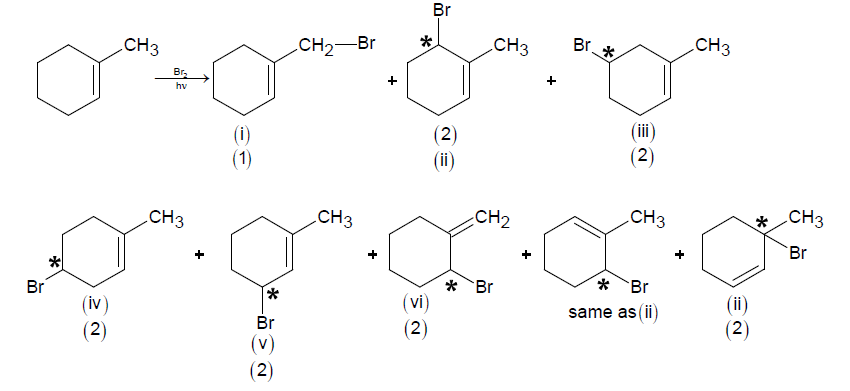
Q17. The maximum number of possible isomers (including stereoisomers) which may be formed on mono-bromination of 1 -methylcyclohex-1-ene using \(\mathrm{Br}_{2}\) and \(\mathrm{UV}\) light is
Answer: 13
Solution:
Q18. In the reaction given below, the total number of atoms having \(s p^{2}\) hybridization in the major product \(\mathbf{P}\) is
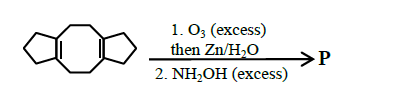
Answer: 12
Solution:
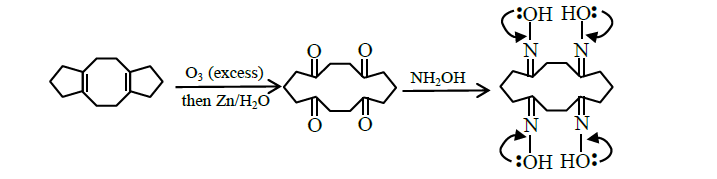
Total 12 atoms are \(\mathrm{sp}^{2}\) hybridised
Q19. The total number of possible isomers for \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right] \mathrm{Br}_{2}\) is ________.
Answer: 6
Solution:
Isomers
(I)\(\quad\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right] \mathrm{Br}_{2} \Rightarrow\) G.I. =2
(II) \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Br}_{2}\right] \mathrm{Cl}_{2} \Rightarrow\) G.I. =2
(III) \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{BrCl}\right] \mathrm{Br} \mathrm{Cl} \Rightarrow\) G.I. =2
I, II, and III are ionization isomers of each other, each having 2 geometrical isomers. The total possible isomers will be 6