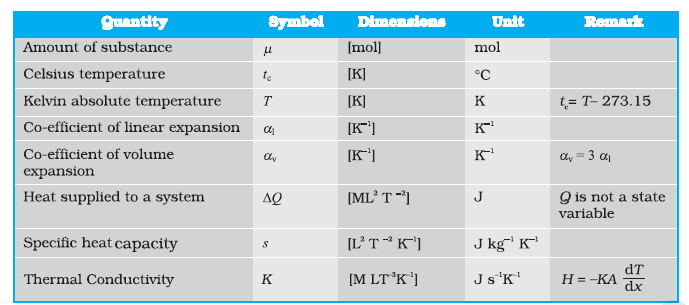
11.11 Summary
- Heat is a form of energy that flows between a body and its surrounding medium by virtue of the temperature difference between them. The degree of hotness of the body is quantitatively represented by temperature.
- A temperature-measuring device (thermometer) makes use of some measurable property (called thermometric property) that changes with temperature. Different thermometers lead to different temperature scales. To construct a temperature scale, two fixed points are chosen and assigned some arbitrary values of temperature. The two numbers fix the origin of the scale and the size of its unit.
- The Celsius temperature \(\left(t_{\mathrm{c}}\right)\) and the Farenheit temperature \(\left(t_{\mathrm{F}}\right)\) are related by
\(
t_{\mathrm{F}}=(9 / 5) t_{\mathrm{C}}+32
\) - The ideal gas equation connecting pressure \((P)\), volume \((V)\) and absolute temperature \((T)\) is :
\(
P V=\mu R T
\)
where \(\mu\) is the number of moles and \(R\) is the universal gas constant. - In the absolute temperature scale, the zero of the scale corresponds to the temperature where every substance in nature has the least possible molecular activity. The Kelvin absolute temperature scale \((T)\) has the same unit size as the Celsius scale \(\left(T_c\right)\), but differs in the origin :
\(
T_{\mathrm{C}}=T-273.15
\) - The coefficient of linear expansion \(\left(\alpha_l\right)\) and volume expansion \(\left(\alpha_v\right)\) are defined by the relations :
\(
\begin{aligned}
& \frac{\Delta l}{l}=\alpha_1 \Delta T \\
& \frac{\Delta V}{V}=\alpha_V \Delta T
\end{aligned}
\)
where \(\Delta l\) and \(\Delta V\) denote the change in length \(l\) and volume \(V\) for a change of temperature \(\Delta T\). The relation between them is :
\(
\alpha_{\mathrm{v}}=3 \alpha_l
\) - The specific heat capacity of a substance is defined by
\(
s=\frac{1}{m} \frac{\Delta Q}{\Delta T}
\)
where \(m\) is the mass of the substance and \(\Delta Q\) is the heat required to change its temperature by \(\Delta T\). The molar specific heat capacity of a substance is defined by
\(
C=\frac{1}{\mu} \frac{\Delta Q}{\Delta T}
\)
where \(\mu\) is the number of moles of the substance. - The latent heat of fusion \((L)\) is the heat per unit mass required to change a substance from solid into liquid at the same temperature and pressure. The latent heat of vaporisation \(\left(L_{\mathrm{s}}\right)\) is the heat per unit mass required to change a substance from liquid to the vapour state without change in the temperature and pressure.
- The three modes of heat transfer are conduction, convection, and radiation.
- In conduction, heat is transferred between neighbouring parts of a body through molecular collisions, without any flow of matter. For a bar of length \(L\) and uniform cross section \(A\) with its ends maintained at temperatures \(T_C\) and \(T_D\), the rate of flow of heat \(H\) is :
\(
H=K A \frac{T_C-T_D}{L}
\)
where \(K\) is the thermal conductivity of the material of the bar. - Newton’s Law of Cooling says that the rate of cooling of a body is proportional to the excess temperature of the body over the surroundings :
\(
\frac{\mathrm{d} Q}{\mathrm{~d} t}=-k\left(T_2-T_1\right)
\)
Where \(T_1\) is the temperature of the surrounding medium and \(T_2\) is the temperature of the body.
POINTS TO PONDER:
- The relation connecting Kelvin temperature \((T)\) and the Celsius temperature \(t_c\)
\(
T=t_c+273.15
\)
and the assignment \(T=273.16 \mathrm{~K}\) for the triple point of water are exact relations (by choice). With this choice, the Celsius temperature of the melting point of water and boiling point of water (both at \(1 \mathrm{~atm}\) pressure) are very close to, but not exactly equal to \(0^{\circ} \mathrm{C}\) and \(100^{\circ} \mathrm{C}\) respectively. In the original Celsius scale, these latter fixed points were exactly at \(0^{\circ} \mathrm{C}\) and \(100^{\circ} \mathrm{C}\) (by choice), but now the triple point of water is the preferred choice for the fixed point because it has a unique temperature. - A liquid in equilibrium with vapour has the same pressure and temperature throughout the system; the two phases in equilibrium differ in their molar volume (i.e. density). This is true for a system with any number of phases in equilibrium.
- Heat transfer always involves temperature difference between two systems or two parts of the same system. Any energy transfer that does not involve temperature difference in some way is not heat.
- Convection involves flow of matter within a fluid due to unequal temperatures of its parts. A hot bar placed under a running tap loses heat by conduction between the surface of the bar and water and not by convection within the water.