Sample Paper
These 20 sample questions are taken from JEE 2021 Entrance Paper (Section-A)
Q1. Which one of the following complexes is violet in colour?
(1) \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\)
(2) \(\left[\mathrm{Fe}(\mathrm{SCN})_{6}\right]^{4-}\)
(3) \(\mathrm{Fe}_{4}\left[\mathrm{Fe}\left(\mathrm{CN}_{6}\right)\right]_{3} \cdot \mathrm{H}_{2} \mathrm{O}\)
(4) \(\left[\mathrm{Fe}(\mathrm{CN})_{5} \mathrm{NOS}^{4}\right.\)
Answer: 4
Q2. Which one of the following is correct for the adsorption of a gas at a given temperature on a solid surface?
(1) \(\Delta \mathrm{H}>0, \Delta \mathrm{S}>0\)
(2) \(\Delta \mathrm{H}>0, \Delta \mathrm{S}<0\)
(3) \(\Delta \mathrm{H}<0, \Delta \mathrm{S}<0\)
(4) \(\Delta \mathrm{H}<0, \Delta \mathrm{S}>0\)
Answer: 3
Q3. Which one of the following when dissolved in water gives coloured solution in nitrogen atmosphere?
(1) \(\mathrm{CuCl}_{2}\)
(2) \(\mathrm{AgCl}\)
(3) \(\mathrm{ZnCl}_{2}\)
(4) \(\mathrm{Cu}_{2} \mathrm{Cl}_{2}\)
Answer: 1
Q4. The major products formed in the following reaction sequence A and B are:
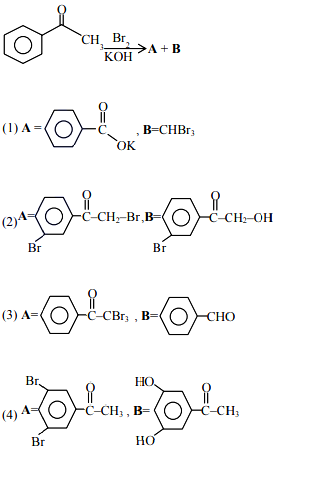
Answer: 1
Q5. The major product formed in the following reaction is:
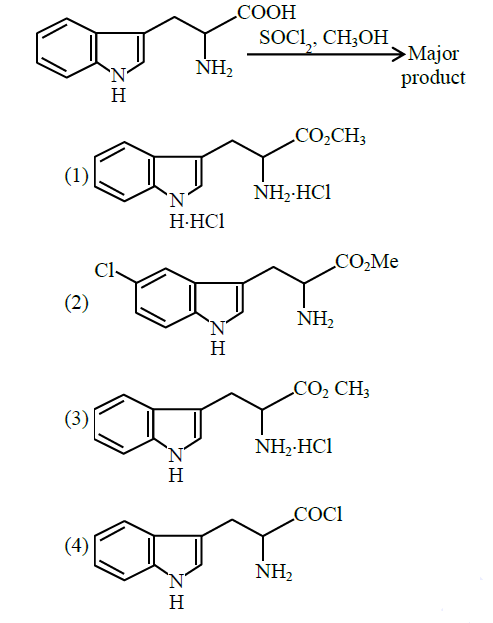
Answer: 3
Q6. The major product formed in the following reaction is:
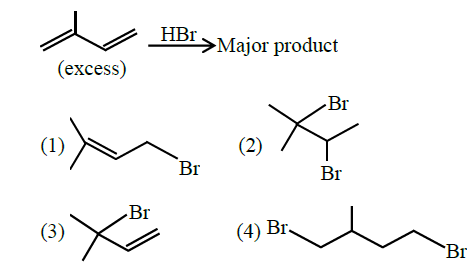
Answer: 1
Q7. The polymer formed on heating Novolac with formaldehyde is:
(1) Bakelite
(2) Polyester
(3) Melamine
(4) Nylon 6,6
Answer: 1
Q8. Given below are two statements :
Statement I: The limiting molar conductivity of \(\mathrm{KCl}\) (strong electrolyte) is higher compared to that of \(\mathrm{CH}_{3} \mathrm{COOH}\) (weak electrolyte).
Statement II: Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1) Statement I is true but Statement II is false.
(2) Statement I is false but Statement II is true.
(3) Both Statement I and Statement II are true.
(4) Both Statement I and Statement II are false.
Answer: 4
Q9. The correct options for products A and B of the following reactions are:
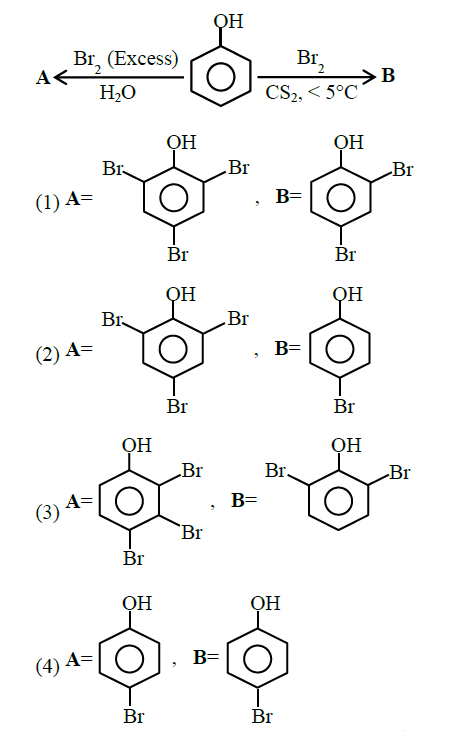
Answer: 2
Q10. The conversion of hydroxyapatite occurs due to presence of \(\mathrm{F}^{-}\)ions in water. The correct formula of hydroxyapatite is:
(1) \(\left[3 \mathrm{Ca}_{3}\left(\mathrm{PO}_{4}\right)_{2} \cdot \mathrm{Ca}(\mathrm{OH})_{2}\right]\)
(2) \(\left[3 \mathrm{Ca}(\mathrm{OH})_{2} \cdot \mathrm{CaF}_{2}\right]\)
(3) \(\left[\mathrm{Ca}_{3}\left(\mathrm{PO}_{4}\right)_{2} \cdot \mathrm{CaF}_{2}\right]\)
(4) \(\left[3 \mathrm{Ca}_{3}\left(\mathrm{PO}_{4}\right)_{2} \cdot \mathrm{CaF}_{2}\right]\)
Answer: 1
Q11. Given below are two statements.
Statement I: In the titration between a strong acid and weak base methyl orange is suitable as an indicator.
Statement II: For titration of acetic acid with NaOH phenolphthalein is not a suitable indicator. In the light of the above statements, choose the most appropriate answer from the options given below:
(1) Statement I is false but Statement II is true
(2) Statement I is true but Statement II is false
(3) Both Statement I and Statement II are true
(4) Both Statement I and Statement II are false
Answer: 2
Q12. Among the following compounds I-IV, which one forms a yellow precipitate on reacting sequentially with (I) \(\mathrm{NaOH}\) (ii) \(\mathrm{dil.}\) \(\mathrm{HNO}_{3}\) (iii) \(\mathrm{AgNO}_{3}\)?
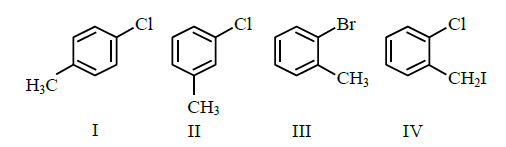
(1) II
(2) IV
(3) I
(4) III
Answer: 2
Q13. Which one of the following methods is most suitable for preparing deionized water?
(1) Synthetic resin method
(2) Clark’s method
(3) Calgon’s method
(4) Permutit method
Answer: 1
Q14. Given below are two statements.
Statement I: The choice of reducing agents for metals extraction can be made by using the Ellingham diagram, a plot of ΔG vs temperature.
Statement II: The value of ΔS increases from left to right in the Ellingham diagram. In the light of the above statements, choose the most appropriate answer from the options given below:
(1) Both Statement I and Statement II are true
(2) Statement I is false but Statement II is true
(3) Both Statement I and Statement II are false
(4) Statement I is true but Statement II is false
Answer: 4
Q15. What are the products formed in sequence when excess of \(\mathrm{CO}_{2}\) is passed in slaked lime?
(1) \(\mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}, \mathrm{CaCO}_{3}\)
(2) \(\mathrm{CaCO}_{3}, \mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}\)
(3) \(\mathrm{CaO}, \mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}\)
(4) \(\mathrm{CaO}, \mathrm{CaCO}_{3}\)
Answer: 2
Solution:
\(\begin{aligned}&\mathrm{Ca}(\mathrm{OH})_{2}+\mathrm{CO}_{2} \longrightarrow \mathrm{CaCO}_{3} \downarrow+\mathrm{H}_{2} \mathrm{O} \\
&\mathrm{CaCO}_{3} \downarrow+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}
\end{aligned}\)
Q16. Given below are two statements.
Statement I: According to Bohr’s model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in positive charges on the nucleus as there is no strong hold on the electron by the nucleus.
Statement II: According to Bohr’s model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in principal quantum number.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1) Both Statement I and Statement II are false
(2) Both Statement I and Statement II are true
(3) Statement I is false but Statement II is true
(4) Statement I is true but Statement II is false
Answer: 3
Solution:
Velocity of electron in Bohr’s atom is given by \(\mathrm{V} \propto \frac{\mathrm{Z}}{\mathrm{n}}\)
\(Z\) = atomic number of the atom, corresponds to \(+v e\) charge so as \(Z\) increase velocity increases so statement-I is wrong. As ‘ \(n\) ‘ decreases velocity increases so statement-II is correct.
Q17. The correct sequential addition of reagents in the preparation of 3 -nitrobenzoic acid from benzene is:
(1) \(\mathrm{Br}_{2} / \mathrm{AlBr}_{3}, \mathrm{HNO}_{3} / \mathrm{H}_{2} \mathrm{SO}_{4}, \mathrm{Mg} /\) ether, \(\mathrm{CO}_{2}, \mathrm{H}_{3} \mathrm{O}^{+}\)
(2) \(\mathrm{Br}_{2} / \mathrm{AlBr}_{3}, \mathrm{NaCN}, \mathrm{H}_{3} \mathrm{O}^{+}, \mathrm{HNO}_{3} / \mathrm{H}_{2} \mathrm{SO}_{4}\)
(3) \(\mathrm{Br}_{2} / \mathrm{AlBr}_{3}, \mathrm{HNO}_{3} / \mathrm{H}_{2} \mathrm{SO}_{4}, \mathrm{NaCN}, \mathrm{H}_{3} \mathrm{O}^{+}\)
(4) \(\mathrm{HNO}_{3} / \mathrm{H}_{2} \mathrm{SO}_{4}, \mathrm{Br}_{2} / \mathrm{AlBr}_{3}, \mathrm{Mg} /\) ether, \(\mathrm{CO}_{2}, \mathrm{H}_{3} \mathrm{O}^{+}\)
Answer: 4
Solution:
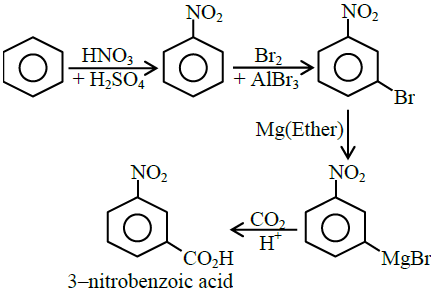
Q18. Given below are two statements.
Statement I: Frenkel defects are vacancy as well as interstitial defects.
Statement II: Frenkel defect leads to colour in ionic solids due to the presence of F-centres.
Choose the most appropriate answer for the statements from the options given below:
(1) Statement I is false but Statement II is true
(2) Both Statement I and Statement II are true
(3) Statement I is true but Statement II is false
(4) Both Statement I and Statement II are false
Answer: 3
Q19. The incorrect statement is:
(1) \(\mathrm{Cl}_{2}\) is more reactive than \(\mathrm{ClF}\).
(2) \(\mathrm{F}_{2}\) is more reactive than \(\mathrm{ClF}\).
(3) On hydrolysis CIF froms HOCl and HF
(4) \(\mathrm{F}_{2}\) is a stronger oxidizing agent than \(\mathrm{Cl}_{2}\) in aqueous solution
Answer: 1
Solution:
(i) Reactivity order :
\(
\mathrm{F}_{2}>\mathrm{ClF} \text { (inter halogen) }>\mathrm{Cl}_{2}\)
(ii) \(\mathrm{ClF}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{HOCl}+\mathrm{HF}\)
(iii) Oxidizing power in aqueous solution
\(
\mathrm{F}_{2}>\mathrm{Cl}_{2}>\mathrm{Br}_{2}>\mathrm{I}_{2}
\)
Q20. Excess of isobutane on reaction with \(\mathrm{Br}_{2}\) in presence of light at \(125^{\circ} \mathrm{C}\) gives which one of the following, as the major product?
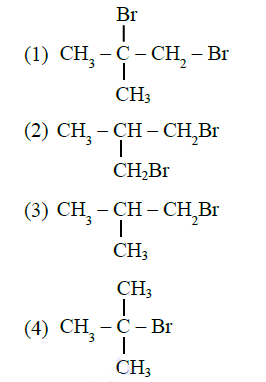
Answer: 4