NEET PYQs
Quiz Summary
0 of 37 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 37 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 37
1. Question
Hot concentrated sulphuric acid is a moderately strong oxidizing agent. Which of the following reactions does not show oxidizing behaviour? [NEET-II 2016]
CorrectIncorrectHint
(d) \(\mathrm{CaF}_{2}+\mathrm{H}_{2} \mathrm{SO}_{4} \rightarrow \mathrm{CaSO}_{4}+2 \mathrm{HF}\)
Here, the oxidation state of every atom remains the same so, it is not a redox reaction. -
Question 2 of 37
2. Question
The pair of compounds that can exist together is [NEET 2014]
CorrectIncorrectHint
(c)Both \(\mathrm{FeCl}_{2}\) and \(\mathrm{SnCl}_{2}\) are reducing agents with low oxidation numbers
-
Question 3 of 37
3. Question
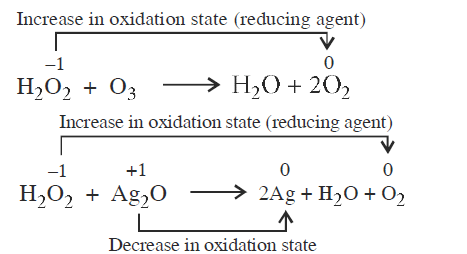
(I) \(\mathrm{H}_{2} \mathrm{O}_{2}+\mathrm{O}_{3} \longrightarrow \mathrm{H}_{2} \mathrm{O}+2 \mathrm{O}_{2}\)
(II) \(\mathrm{H}_{2} \mathrm{O}_{2}+\mathrm{Ag}_{2} \mathrm{O} \longrightarrow 2 \mathrm{Ag}+\mathrm{H}_{2} \mathrm{O}+\mathrm{O}_{2}\)
Role of hydrogen peroxide in the above reactions is respectively [NEET 2014]CorrectIncorrectHint
(c)
\(\mathrm{H}_{2} \mathrm{O}_{2}\) acts as reducing agent in all those reactions in which \(\mathrm{O}_{2}\) is evolved
-
Question 4 of 37
4. Question
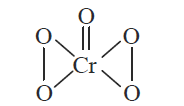
In acidic medium, \(\mathrm{H}_{2} \mathrm{O}_{2}\) changes \(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) to \(\mathrm{CrO}_{5}\) which has two (-O-O-) bonds. Oxidation state of \(\mathrm{Cr}\) in \(\mathrm{CrO}_{5}\) is [NEET 2014]
CorrectIncorrectHint
(c) \(\mathrm{CrO}_{5}\) has butterfly structure having two peroxo bonds.
Peroxo oxygen has \(-1\) oxidation state.
Let oxidation state of \(\mathrm{Cr}\) be ‘ \(x\) ‘
\(
\mathrm{CrO}_{5}: x+4(-1)+1(-2)=0 \Rightarrow x=+6
\)
-
Question 5 of 37
5. Question
When \(\mathrm{Cl}_{2}\) gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from [NEET 2012]
CorrectIncorrectHint
(b)
\(
\stackrel{0}{3 \mathrm{Cl}_{2}}+\underset{\text { (hot and conc.) }}{6 \mathrm{NaOH}} \longrightarrow 5 \mathrm{Na} \stackrel{-1}{\mathrm{Cl}}+\stackrel{+5}{\mathrm{NaCl} \mathrm{O}_3}+3 \mathrm{H}_2 \mathrm{O}
\)
This is an example of disproportionation reaction and oxidation state of chlorine changes from 0 to \(-1\) and \(+5\) -
Question 6 of 37
6. Question
A mixture of potassium chlorate, oxalic acid and sulphuric acid is heated. During the reaction which element undergoes maximum change in the oxidation number? [NEET 2012]
CorrectIncorrectHint
(c) \(
{\mathrm{~K}} \stackrel{+5}{\mathrm{Cl}} \mathrm{O}_3+\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4+\mathrm{H}_2 \stackrel{+6}{\mathrm{S}} \mathrm{{O}}_4 \rightarrow {\mathrm{~K_2}} \stackrel{+6}{\mathrm{~S}} \mathrm{{O}}_4+\stackrel{-1}{\mathrm{KCl}}+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}
\)
i.e. maximum change in oxidation number is observed in \(\mathrm{Cl}\) ( +5 to -1). -
Question 7 of 37
7. Question
Oxidation numbers of \(\mathrm{P}\) in \(\mathrm{PO}_{4}^{3-}\), of \(\mathrm{S}\) in \(\mathrm{SO}_{4}^{2-}\) and that of \(\mathrm{Cr}\) in \(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) are respectively [NEET 2009]
CorrectIncorrectHint
(d) Let oxidation number of \(\mathrm{P}\) in \(\mathrm{PO}_{4}^{3-}\) be \(x\).
\(
\therefore \quad x+4(-2)=-3 \quad \Rightarrow x=+5
\)
Let oxidation number of \(\mathrm{S}\) in \(\mathrm{SO}_{4}^{2-}\) be \(y\).
\(
\therefore \quad y+4(-2)=-2 \quad \Rightarrow \quad y=+6
\)Let oxidation number of \(\mathrm{Cr}\) in \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\) be \(z\).
\(
\therefore \quad 2 z+7(-2)=-2 \Rightarrow z=+6
\) -
Question 8 of 37
8. Question
Number of moles of \(\mathrm{MnO^{-}}_{4}\) required to oxidize one mole of ferrous oxalate completely in acidic medium will be [NEET 2008]
CorrectIncorrectHint
(d) \(\left[5 e^{-}+\mathrm{MnO}_{4}^{-}+8 \mathrm{H}^{+} \rightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_{2} \mathrm{O}\right.\)..(i) \(] \times 2\)
\(\left[\mathrm{C}_{2} \mathrm{O}_{4}^{2-} \rightarrow 2 e^{-}+2 \mathrm{CO}_{2} \ldots\right.\) (ii) \(] \times 5\)
\(2 \mathrm{MnO}_{4}^{-}+16 \mathrm{H}^{+}+5 \mathrm{C}_{2} \mathrm{O}_{4}^{2-} \rightarrow 2 \mathrm{Mn}^{2+}+10 \mathrm{CO}_{2}\)
2 moles of \(\mathrm{MnO}_{4}^{-}\)required to oxidise 5 moles of oxalate.
\(\therefore\) Number of moles of \(\mathrm{MnO}_{4}^{-}\)required to oxidise 1 mole of oxalate \(=2 / 5=0.4\) -
Question 9 of 37
9. Question
Which is the best description of the behaviour of bromine in the reaction given below?
\(
\mathrm{H}_{2} \mathrm{O}+\mathrm{Br}_{2} \rightarrow \mathrm{HOBr}+\mathrm{HBr}
\) [NEET 2004]CorrectIncorrectHint
(b) \(
\mathrm{H}_2 \mathrm{O}+\stackrel{0}{\mathrm{B{r_2}}} \rightarrow \mathrm{HO}\stackrel{+1}{\mathrm{Br}}+\mathrm{H} \stackrel{-1}{\mathrm{Br}}
\)
In the above reaction the oxidation number of \(\mathrm{Br}_{2}\) increases from zero (in \(\mathrm{Br}_{2}\) ) to \(+1\) (in \(\mathrm{HOBr}\) ) and decreases from zero (in \(\mathrm{Br}_{2}\) ) to \(-1\) (in \(\mathrm{HBr}\) ). Thus \(\mathrm{Br}_{2}\) is oxidised as well as reduced and hence it is a redox reaction. -
Question 10 of 37
10. Question
The oxidation states of sulphur in the anions \(\mathrm{SO}_{3}{ }^{2-}, \mathrm{S}_{2} \mathrm{O}_{4}{ }^{2-}\) and \(\mathrm{S}_{2} \mathrm{O}_{6}{ }^{2-}\) follow the order [NEET 2003]
CorrectIncorrectHint
(a) \(\mathrm{SO}_{3}{ }^{2-}: x+(-2) 3=-2\)
or \(x-6=-2\) or \(x=+4\)
\(\mathrm{S}_{2} \mathrm{O}_{4}{ }^{2-}: 2 x+(-2) 4=-2\)
or \(2 x-8=-2\) or \(2 x=+6 \therefore x=+3\)
\(\mathrm{S}_{2} \mathrm{O}_{6}^{2-}: 2 x+(-2) 6=-2\)
or, \(2 x-12=-2\) or \(2 x=+10 \therefore x=+5\)
Oxidation states follow the order:
\(
\mathrm{S}_{2} \mathrm{O}_{4}{ }^{2-} \lt \mathrm{SO}_{3}{ }^{2-} \lt \mathrm{S}_{2} \mathrm{O}_{6}{ }^{2-}
\) -
Question 11 of 37
11. Question
Oxidation state of \(\mathrm{Fe}\) in \(\mathrm{Fe}_{3} \mathrm{O}_{4}\) is [AIPMT 1999]
CorrectIncorrectHint
\(
\text { (d) } \mathrm{Fe}_{3} \mathrm{O}_{4} \rightarrow 3 x+4(-2)=0 \Rightarrow x=+\frac{8}{3}
\) -
Question 12 of 37
12. Question
Which of the following is redox reaction? [AIPMT 1997]
CorrectIncorrectHint
(b) Redox reactions are those chemical reactions which involve transfer of electrons from one chemical species to another.
-
Question 13 of 37
13. Question
The oxide, which cannot act as a reducing agent is [AIPMT 1995]
CorrectIncorrectHint
(a) Since carbon is in maximum state of \(+4\), therefore carbon dioxide \(\left(\mathrm{CO}_{2}\right)\) cannot act as a reducing agent
-
Question 14 of 37
14. Question
Which substance is serving as a reducing agent in the following reaction? [AIPMT 1994]
\(14 \mathrm{H}^{+}+\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+3 \mathrm{Ni} \rightarrow 7 \mathrm{H}_{2} \mathrm{O}+2 \mathrm{Cr}^{3+}+3 \mathrm{Ni}^{2+}\)CorrectIncorrectHint
(d) Since the oxidation number of Ni increases from 0 to 2 , therefore it acts as a reducing agent.
-
Question 15 of 37
15. Question
The oxidation state of \(\mathrm{I}\) in \(\mathrm{H}_{4} \mathrm{IO}_{6}^{-}\)is [AIPMT 1994]
CorrectIncorrectHint
(c) Let \(x=\) Oxidation state of \(\mathrm{I}\). Since oxidation state of \(\mathrm{H}=+1\) and oxidation state of \(\mathrm{O}=-2\), therefore for \(\mathrm{H}_{4} \mathrm{IO}_{6}^{-}\), we get
\(
(4 \times 1)+x+(6 \times-2)=-1
\)\(
\text { or } x=+7
\) -
Question 16 of 37
16. Question
The oxidation state of \(\mathrm{Cr}\) in \(\mathrm{CrO}_{5}\) is [NEET (Odisha) 2019]
CorrectIncorrectHint
(c)

Oxidation Number of \(\mathrm{Cr}\) in \(\mathrm{CrO}_5\)
- The oxidation number of \(\mathrm{Cr}\) be \(\mathrm{x}\).
- Four oxygen atoms are attached to \(\mathrm{Cr}\) through a single bond. The oxygen is in peroxide form. Hence oxidation state of the oxygen atom, in this case, is -1 .
- Another oxygen atom is attached through a double bond and this has an oxidation number -2 .
\(
\begin{array}{c}
x+(-2)+4(-1)=0 \\
x=+6
\end{array}
\)
Hence, the oxidation number of \(\mathrm{Cr}\) in \(\mathrm{CrO}_5\) is +6 .
-
Question 17 of 37
17. Question
The correct option for a redox couple is: [NEET 2023 Manipur]
CorrectIncorrectHint
(c) Redox couple is both the reduced and oxidised form involve same element.
-
Question 18 of 37
18. Question
On balancing the given redox reaction,
\(
\mathrm{aCr}_2 \mathrm{O}_7^{2-}+\mathrm{bSO}_3^{2-}(\mathrm{aq})+\mathrm{cH}^{+}(\mathrm{aq}) \rightarrow 2 \mathrm{aCr}^{3+}(\mathrm{aq})+\mathrm{bSO}_4^{2-}(\mathrm{aq})+\frac{\mathrm{c}}{2} \mathrm{H}_2 \mathrm{O}(\ell)
\)
the coefficients \(\mathrm{a}, \mathrm{b}\) and \(\mathrm{c}\) are found to be, respectively – [NEET 2023]CorrectIncorrectHint
(d) Reaction has to be balanced in acidic medium ‘O’ atoms are balanced by adding \(\mathrm{H}_2 \mathrm{O}\) and then \(\mathrm{H}\)-atom is balanced by adding \(\mathrm{H}^{+}\)ions and charge is balanced by \(e^{\ominus}\).
Oxidation: \(\left.\mathrm{SO}_3^{2-}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{SO}_4^{2-}+2 \mathrm{H}^{+}+2 e^{\ominus}\right] \times 3\)
Reduction: \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 e^{\ominus} \rightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O}\)
\(
\mathrm{Cr}_2 \mathrm{O}_7^{2-}+3 \mathrm{SO}_3^{2-}+8 \mathrm{H}^{\oplus} \rightarrow 2 \mathrm{Cr}^{3+}+3 \mathrm{SO}_4^{2-}+4 \mathrm{H}_2 \mathrm{O}
\)
Comparing with given equation
\(
\begin{array}{l}
\mathrm{a}=1 \\
\mathrm{~b}=3 \\
\mathrm{c}=8
\end{array}
\) -
Question 19 of 37
19. Question
Which of the following reactions is a decomposition redox reaction? [NEET 2022 Phase 2]
CorrectIncorrectHint
(b) Decomposition redox reaction leads to breakdown of a compound into two or more compounds at least one of which must be in the elemental state with change in oxidation number.
\(
2 \mathrm{~Pb}\left(\mathrm{NO}_3^{2-}\right)_2(\mathrm{~s}) \rightarrow 2 \mathrm{PbO}(\mathrm{s})+4 \mathrm{NO}_2(\mathrm{~g})+\stackrel{0}{\mathrm{O}_2}(\mathrm{~g})
\) -
Question 20 of 37
20. Question
What is the change in oxidation number of carbon in the following reaction?
\(
\mathrm{CH}_4(\mathrm{~g})+4 \mathrm{Cl}_2(\mathrm{~g}) \rightarrow \mathrm{CCl}_4(\mathrm{l})+4 \mathrm{HCl}(\mathrm{g})
\) [NEET 2020]CorrectIncorrectHint
(b)
\(
\stackrel{-4}{\mathrm{CH}_4}(\mathrm{~g})+4 \mathrm{Cl}_2(\mathrm{~g}) \rightarrow \stackrel{+4}{\mathrm{CCl}_4}(l)+4 \mathrm{HCl}(\mathrm{g})
\)
Change in oxidation state of carbon is from -4 to +4. -
Question 21 of 37
21. Question
Which of the following reactions are disproportionation reaction?
(a) \(2 \mathrm{Cu}^{+} \rightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}^0\)
(b) \(3 \mathrm{MnO}_4^{2+}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_4^{-}+\mathrm{MnO}_2+2 \mathrm{H}_2 \mathrm{O}\)
(c) \(2 \mathrm{KMnO}_4 \xrightarrow{\Delta} \mathrm{K}_2 \mathrm{MnO}_4+\mathrm{MnO}_2+\mathrm{O}_2\)
(d) \(2 \mathrm{MnO}_4^{-}+3 \mathrm{Mn}^{2+}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 5 \mathrm{MnO}_2+4 \mathrm{H}^{\oplus}\)
Select the correct option from the following : [NEET 2019]CorrectIncorrectHint
(b) In a disproportionation reaction, same substance undergoes oxidation (increase in oxidation number) and reduction (decrease in oxidation number forming two different products.
\(
\text { (a) } 2 \mathrm{Cu}^{+1} \longrightarrow \mathrm{Cu}^{+2}+\mathrm{Cu}^0 \text { (Disproportionation) }
\)
\(
\text { (b) } 3 \stackrel{+6}{\mathrm{Mn}} \mathrm{O}_4^{2-}+4 \mathrm{H}^{+} \longrightarrow 2\stackrel{+7}{\mathrm{M}} \mathrm{nO}_4^{-}+\stackrel{+4}{\mathrm{M}} \mathrm{nO}_2+2 \mathrm{H}_2 \mathrm{O} \text { (Disproportionation) }
\)
\(
\text { (c) } 2 \mathrm{K} \stackrel{+7}{\mathrm{M}}{nO}_4 \stackrel{\Delta}{\longrightarrow} \mathrm{K}_2 \stackrel{+6}{\mathrm{M}} \mathrm{nO}_4+\stackrel{+4}{\mathrm{M}} \mathrm{nO}_2+\stackrel{0}{\mathrm{O_2}}
\)
\(
\text { (d) } 2 \mathrm{K} \stackrel{+7}{\mathrm{M}} {\mathrm{nO}}_4^{-}+3 \stackrel{+2}{\mathrm{M}}\mathrm{n}^{2+}+2 \mathrm{H}_2 \mathrm{O}\longrightarrow \stackrel{+4}{\mathrm{M}}\mathrm{nO}_2+4 \mathrm{H}^{+}
\) -
Question 22 of 37
22. Question
For the redox reaction,
\(
\mathrm{MnO}_4{ }^{-}+\mathrm{C}_2 \mathrm{O}_4{ }^{2-}+\mathrm{H}^{+} \rightarrow \mathrm{Mn}^{2+}+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}
\)
The correct coefficients of the reactants for the balanced equation are [NEET 2018]CorrectIncorrectHint
(b) The correct balanced equation is
\(
2 \mathrm{MnO}_4{ }^{-}+5 \mathrm{C}_2 \mathrm{O}_4{ }^{2-}+16 \mathrm{H}^{+} \rightarrow 2 \mathrm{Mn}^{2+}+10 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}
\) -
Question 23 of 37
23. Question
The correct order of \(\mathrm{N}\)-compounds in its decreasing order of oxidation states is [NEET 2018]
CorrectIncorrectHint
(a)
\(
\stackrel{+5}{\mathrm{~N}} \mathrm{O}_3, \stackrel{+2}{\mathrm{~N}} \mathrm{O}, \stackrel{0}{\mathrm{~N}}, \stackrel{-3}{\mathrm{~N}} \mathrm{H}_4 \mathrm{Cl}
\) -
Question 24 of 37
24. Question
\(\mathrm{Zn}\) gives \(\mathrm{H}_2\) gas with \(\mathrm{H}_2 \mathrm{SO}_4\) and \(\mathrm{HCl}\) but not with \(\mathrm{HNO}_3\) because [AIPMT 2002]
CorrectIncorrectHint
(d) Zinc gives \(\mathrm{H}_2\) gas with dil \(\mathrm{H}_2 \mathrm{SO}_4 / \mathrm{HCl}\) but not with \(\mathrm{HNO}_3\) because in \(\mathrm{HNO}_3, \mathrm{NO}_3^{-}\)ion is reduced and give \(\mathrm{NH}_4 \mathrm{NO}_3, \mathrm{~N}_2 \mathrm{O}, \mathrm{NO}\) and \(\mathrm{NO}_2\)
Note:
\(
\begin{array}{r}
[\mathrm{Zn}+\underset{(\text { nearly } 6 \%)}{2 \mathrm{HNO}_3}\left.\longrightarrow \mathrm{Zn}\left(\mathrm{NO}_3\right)_3+2 \mathrm{H}\right] \times 4
\end{array}
\)
\(
\begin{array}{l}
\mathrm{HNO}_3+8 \mathrm{H} \longrightarrow \mathrm{NH}_3+3 \mathrm{H}_2 \mathrm{O} \\
\mathrm{NH}_3+\mathrm{HNO}_3 \longrightarrow \mathrm{NH}_4 \mathrm{NO}_3 \\
4 \mathrm{Zn}+10 \mathrm{HNO}_3 \longrightarrow 4 \mathrm{Zn}\left(\mathrm{NO}_3\right)_2+\mathrm{NH}_4 \mathrm{NO}_3+3 \mathrm{H}_2 \mathrm{O}
\end{array}
\)
\(\mathrm{Zn}\) is above of hydrogen in electrochemical series. So, \(\mathrm{Zn}\) displaces \(\mathrm{H}_2\) from dilute \(\mathrm{H}_2 \mathrm{SO}_4\) and \(\mathrm{HCl}\) with liberation of \(\mathrm{H}_2\).
\(
\mathrm{Zn}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{ZnSO}_4+\mathrm{H}_2
\) -
Question 25 of 37
25. Question
Which of the following involves a redox reaction? [AIPMT 1997]
CorrectIncorrectHint
(c)
\(
\text { (a) } 2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \underset{\text { (neutralization) }}{\mathrm{Na}_2 \mathrm{SO}_4+2 \mathrm{H}_2 \mathrm{O}}
\)
\(
\text { (b) } \quad \stackrel{0}{3 \mathrm{O}_2} \xrightarrow{\text { Light }} \stackrel{0}2{\mathrm{O}_3} \text { (not redox reaction) }
\)
\(
\text { (c) } \underset{0}{\mathrm{N}_2}+\underset{0}{\mathrm{O}_2} \xrightarrow{\text { Light }} \underset{+2-2}{2 \mathrm{NO}} \text { (redox reaction) }
\)
here oxidation of \(\mathrm{N}_2\) \& reduction of \(\mathrm{O}_2\) is taking place
\(
\text { (d) } \mathrm{H}_2 \mathrm{O} \text { (l) } \xrightarrow{\Delta} \mathrm{H}_2 \mathrm{O} \text { (g) (not redox reaction) }
\) -
Question 26 of 37
26. Question
The loss of electron is termed as [AIPMT 1995]
CorrectIncorrectHint
(a) Losing of electron is called oxidation.
-
Question 27 of 37
27. Question
A compound contains atoms of three elements \(A, B\) and \(C\). If the oxidation number of \(A\) is \(+2, B\) is +5 , and that of \(C\) is -2 , the possible formula of the compound is : [AIPMT 2000]
CorrectIncorrectHint
(b) Oxidation number of a compound must be 0 . Using the values for \(A, B\) and \(C\) in the four options we find that \(A_3\left(B C_4\right)_2\) is the answer.
Check: \((+2) 3+[(+5)+4(-2)] 2=6+(5-8) 2=0\) -
Question 28 of 37
28. Question
The oxidation number of phosphorus in pyrophosphoric acid is [AIPMT 1999]
CorrectIncorrectHint
(d) Pyrophosphoric acid \(\mathrm{H}_4 \mathrm{P}_2 \mathrm{O}_7\)
Let oxidation state of phosphorus is \(x\)
\(
\begin{array}{l}
(4 \times 1+(-2) \times 7+2 x)=0 \\
\therefore 2 x=10 \text { or } x=+5
\end{array}
\) -
Question 29 of 37
29. Question
The oxidation number of chromium in potassium dichromate is [AIPMT 1988,1995]
CorrectIncorrectHint
(a) Let \(x=\) oxidation no. of \(\mathrm{Cr}\) in \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\).
\(
\therefore(2 \times 1)+(2 \times x)+7(-2)=0
\)
or \(2+2 x-14=0\) or \(x=+6\). -
Question 30 of 37
30. Question
Phosphorus has the oxidation state of +3 in [AIPMT 1994]
CorrectIncorrectHint
(a) O.N. of \(\mathrm{P}\) in \(\mathrm{H}_3 \mathrm{PO}_3\) (phosphorous acid)
\(3 \times 1+x+3 \times(-2)=0\) or \(x=+3\)
In orthophosphoric acid \(\left(\mathrm{H}_3 \mathrm{PO}_4\right)\) O.N. of \(\mathrm{P}\) is +5 , in hypophosphorous acid \(\left(\mathrm{H}_3 \mathrm{PO}_2\right)\) it is +1 while in metaphosphoric acid \(\left(\mathrm{HPO}_3\right)\), it is +5 . -
Question 31 of 37
31. Question
Consider the change in oxidation state of bromine corresponding to different emf values as shown in the diagram below: [NEET 2018]
\(
\mathrm{BrO}_4^{-} \xrightarrow{1.82 \mathrm{~V}} \mathrm{BrO}_3^{-} \xrightarrow{1.5 \mathrm{~V}}\mathrm{HBrO}\xrightarrow{1.595 \mathrm{~V}}\mathrm{Br}_2\xrightarrow{1.0652 \mathrm{~V}}\mathrm{Br}^{-}
\)
Then the species undergoing disproportionation isCorrectIncorrectHint
(c) Calculate \(E_{\text {cell }}^{\circ}\) corresponding to each compound undergoing disproportionation reaction. The reaction for which \(E_{\text {cell }}^{\circ}\) comes out + ve is spontaneous.
\(
\begin{array}{l}
\mathrm{HBrO} \longrightarrow \mathrm{Br}_2; E^{\circ}=1.595 \mathrm{~V}, \mathrm{SRP} \text { (cathode) } \\
\mathrm{HBrO} \longrightarrow \mathrm{BrO}_3^{-}; E^{\circ}=-1.5 \mathrm{~V}, \mathrm{SOP} \text { (anode) }
\end{array}
\)
\(
2 \mathrm{HBrO} \longrightarrow \mathrm{Br}_2+\mathrm{BrO}_3^{-}
\)
\(
\begin{aligned}
E_{\text {cell }}^{\circ} & =\mathrm{SRP}(\text { cathode) }-\mathrm{SRP} \text { (anode) } \\
& =1.595-1.5 \\
& =0.095 \mathrm{~V} \\
E_{\text {cell }}^{\circ}>0 \Rightarrow & \Delta G^{\circ}<0 \text { [spontaneous] }
\end{aligned}
\)
Note: Reaction in (d) involves comproportionation or synproportionation. When two reactants, each containing the same element but with a different oxidation number, form a product in which the element involved reach the same oxidation number.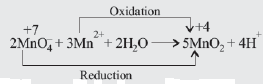
It is opposite to disproportionation. -
Question 32 of 37
32. Question
The following redox reaction is balanced by which set of coefficients? [AIPMT 1999]
\(
\mathrm{aZn}+\mathrm{bNO}_3^{-}+\mathrm{cH}^{+} \rightarrow \mathrm{dNH}_4^{+}+\mathrm{eH}_2 \mathrm{O}+\mathrm{fZn}^{2+}
\)
\(\begin{array}{lllllll} & \mathbf{a} & \mathbf{b} & \mathbf{c} & \mathbf{d} & \mathbf{e} & \mathbf{f} \\ \text { (a) } & 1 & 1 & 10 & 1 & 3 & 1 \\ \text { (b) } & 2 & 2 & 10 & 2 & 3 & 2 \\ \text { (c) } & 4 & 2 & 10 & 1 & 3 & 4 \\ \text { (d) } & 4 & 1 & 10 & 1 & 3 & 4 \end{array}\)CorrectIncorrectHint
(d)
\(
\mathrm{Zn} \rightarrow \mathrm{Zn}^{+2}+2 \mathrm{e}^{-} \dots(1)
\)
\(
8 \mathrm{e}^{-}+10 \mathrm{H}^{+}+\mathrm{NO}_3^{-} \rightarrow \mathrm{NH}_4^{+}+3 \mathrm{H}_2 \mathrm{O} \dots(2)
\)
operate eq. (1) \(\times 4+\) eq. (2) \(\times 1\)
\(
4 \mathrm{Zn}+10 \mathrm{H}^{+}+\mathrm{NO}_3^{-} \rightarrow 4 \mathrm{Zn}^{2+}+\mathrm{NH}_4^{+}+3 \mathrm{H}_2 \mathrm{O}
\) -
Question 33 of 37
33. Question
In which of the following reactions, there is no change in valency? [AIPMT 1994]
CorrectIncorrectHint
(c)
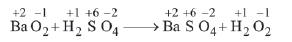
In this reaction, none of the elements undergoes a change in oxidation number or valency.
-
Question 34 of 37
34. Question
The standard electrode potential \(\left(\mathrm{E}^{\circ}\right)\) values of \(\mathrm{Al}^{3+} / \mathrm{Al}, \mathrm{Ag}^{+} / \mathrm{Ag}, \mathrm{K}^{+} / \mathrm{K}\) and \(\mathrm{Cr}^{3+} / \mathrm{Cr}\) are \(-1.66 \mathrm{~V}\), \(0.80 \mathrm{~V},-2.93 \mathrm{~V}\) and \(-0.74 \mathrm{~V}\), respectively. The correct decreasing order of reducing power of the metal is [NEET Odisha 2019]
CorrectIncorrectHint
(c) Lesser is the reduction potential greater is the reducing power.
Reducing power : \(\mathrm{K}>\mathrm{Al}>\mathrm{Cr}>\mathrm{Ag}\) -
Question 35 of 37
35. Question
Standard reduction potentials of the half reactions are given below :
\(
\begin{array}{l}
\mathrm{F}_2(\mathrm{~g})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{~F}^{-}(\mathrm{aq}) ; E^{\circ}=+2.85 \mathrm{~V} \\
\mathrm{Cl}_2(\mathrm{~g})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cl}^{-}(\mathrm{aq}) ; E^{\circ}=+1.36 \mathrm{~V} \\
\mathrm{Br}_2(\mathrm{l})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Br}^{-}(\mathrm{aq}) ; E^{\circ}=+1.06 \mathrm{~V} \\
\mathrm{I}_2(\mathrm{~s})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{I}^{-}(\mathrm{aq}) ; E^{\circ}=+0.53 \mathrm{~V}
\end{array}
\)
The strongest oxidising and reducing agents respectively are : [NEET 2012 M]CorrectIncorrectHint
(a) \(\mathrm{F}_2\) is the strongest oxidising agent as it has highest reduction potential while \(\mathrm{I}^{-}\)is the strongest reducing agent since it has lowest reduction potential.
Higher the value of reduction potential, higher will be the oxidising power whereas the lower the value of reduction potential higher will be the reducing power. -
Question 36 of 37
36. Question
The oxidation states not shown by Mn in given reaction is :
\(
3 MnO _4^{2-}+4 H ^{+} \longrightarrow 2 MnO _4^{-}+ MnO _2+2 H _2 O
\)
A. +6
B. +2
C. +4
D. +7
E. +3
Choose the most appropriate answer from the options given below : [NEET 2024 (Re-Examination)]CorrectIncorrectHint
(d)
\(
\begin{aligned}
&\text { In the following reaction }\\
&3 MnO _4^{2-}+4 H ^{+} \longrightarrow 2 MnO _4^{-}+ MnO _2+2 H _2 O
\end{aligned}
\)
\(
\begin{array}{|c|l|}
\hline \text { Oxidation state of } Mn & \text { Species } \\
\hline+6 & MnO _4^{2-} \\
\hline+7 & MnO _4^{-} \\
\hline+4 & MnO _2 \\
\hline
\end{array}
\)
\(
\text { So }+2 \text { and }+3 \text { oxidation state is not shown by } Mn \text {. }
\) -
Question 37 of 37
37. Question
Which reaction is NOT a redox reaction? [NEET 2024]
CorrectIncorrectHint
(d)