NEET PYQs
Quiz Summary
0 of 134 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 134 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
- 101
- 102
- 103
- 104
- 105
- 106
- 107
- 108
- 109
- 110
- 111
- 112
- 113
- 114
- 115
- 116
- 117
- 118
- 119
- 120
- 121
- 122
- 123
- 124
- 125
- 126
- 127
- 128
- 129
- 130
- 131
- 132
- 133
- 134
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 134
1. Question
Which of the following pairs of compounds is isoelectronic and isostructural? [NEET 2017]
CorrectIncorrectHint
(b) The compounds \(\mathrm{IBr}_{2}^{-}\)and \(\mathrm{XeF}_{2}\) are isostructural. Both have the same number of valence electrons. Both have linear geometry as they have 3 lone pairs and 2 bond pairs of electrons. Electron pair geometry is trigonal bipyramidal and molecular geometry is linear.
Note: Isoelectronic compounds have the same number of electrons. This includes core electrons and valence electrons.
-
Question 2 of 134
2. Question
The species, having bond angles of \(120^{\circ}\) is [NEET 2017]
CorrectIncorrectHint
(c) \(\mathrm{BCl}_{3}\)-Trigonal planar, \(s p^{2}\)-hybridised, \(120^{\circ}\) angle.
-
Question 3 of 134
3. Question
Which one of the following pairs of species have the same bond order? [NEET 2017]
CorrectIncorrectHint
(b) Molecular orbital electronic configurations and bond order values are
\(\mathrm{O}_{2}: \sigma 1 s^{2}, \sigma^{*} 1 s^{2}, \sigma 2 s^{2}, \sigma^{*} 2 s^{2}, \sigma 2 p_{z}^{2}, \pi 2 p_{x}^{2}=\pi 2 p_{y}^{2}\)
\(\pi^{*} 2 p_{x}^{1}=\pi^{*} 2 p_{y}^{1}\)
B.O. \(=\frac{1}{2}\left(N_{b}-N_{a}\right)=\frac{1}{2}(10-6)=2\)
\(\mathrm{NO}^{+}: \sigma 1 s^{2}, \sigma^{*} 1 s^{2}, \sigma 2 s^{2}, \sigma^{*} 2 s^{2}, \sigma 2 p_{z}^{2}, \pi 2 p_{x}^{2}=\pi 2 p_{y}^{2}\)
B. O. \(=\frac{1}{2}(10-4)=3\)
\(\mathrm{CN}^{-}: \sigma 1 s^{2}, \sigma^{*} 1 s^{2}, \sigma 2 s^{2}, \sigma^{*} 2 s^{2}, \pi 2 p_{x}^{2}=\pi 2 p_{y}^{2}, \sigma 2 p_{z}^{2}\)
B.O. \(=\frac{1}{2}(10-4)=3\)
\(\mathrm{CO}: \sigma 1 s^{2}, \sigma^{*} 1 s^{2}, \sigma 2 s^{2}, \sigma^{*} 2 s^{2}, \pi 2 p_{x}{ }^{2}=\pi 2 p_{y}{ }^{2}, \sigma 2 p_{z}{ }^{2}\)
B. O. \(=\frac{1}{2}(10-4)=3\)
\(N_{2}: \sigma 1 s^{2}, \sigma^{*} 1 s^{2}, \sigma 2 s^{2}, \sigma^{*} 2 s^{2}, \pi 2 p_{x}^{2}=\pi 2 p_{y}^{2}, \sigma 2 p_{z}^{2}\)
B. O. \(=\frac{1}{2}(10-4)=3\)
\(\mathrm{O}_{2}^{-}=\sigma 1 s^{2}, \sigma^{*} 1 s^{2}, \sigma 2 s^{2}, \sigma^{*} 2 s^{2}, \sigma 2 p_{z}^{2}, \pi 2 p_{x}^{2}\)
\(=\pi 2 p_{y}{ }^{2}, \pi^{*} 2 p_{x}{ }^{2}=\pi^{*} 2 p_{y}{ }^{1}\)
B.O. \(=\frac{1}{2}(10-7)=1.5\)
\(\mathrm{NO}: \sigma 1 s^{2}, \sigma^{*} 1 s^{2}, \sigma 2 s^{2}, \sigma^{*} 2 s^{2}, \sigma 2 p_{z}^{2}, \pi 2 p_{x}^{2}=\pi 2 p_{y}^{2}\),
\(\pi^{*} 2 p_{x}^{1}\)
B.O. \(=\frac{1}{2}(10-5)=2.5\) -
Question 4 of 134
4. Question
Which one of the following compounds shows the presence of intramolecular hydrogen bond? [NEET-II 2016]
CorrectIncorrectHint
(c) \(\mathrm{H}_{2} \mathrm{O}_{2}, \mathrm{HCN}\) and conc. \(\mathrm{CH}_{3} \mathrm{COOH}\) form intermolecular hydrogen bonding while cellulose has intramolecular hydrogen bonding.
-
Question 5 of 134
5. Question
The hybridizations of atomic orbitals of nitrogen in \(\mathrm{NO}_{2}^{+}, \mathrm{NO}_{3}^{-}\)and \(\mathrm{NH}_{4}^{+}\)respectively are [NEET-II 2016]
CorrectIncorrectHint
\(\text { (c) } X=\frac{1}{2}(V E+M A-c+a)\)
For \(\mathrm{NO}_{2}^{+}, X=\frac{1}{2}(5+0-1)=2\) i.e., \(s p\) hybridisation
For \(\mathrm{NO}_{3}^{-}, X=\frac{1}{2}(5+0+1)=3\) i.e., \(s p^{2}\) hybridisation
For \(\mathrm{NH}_{4}^{+}, X=\frac{1}{2}(5+4-1)=4\) i.e., \(s p^{3}\) hybridisation -
Question 6 of 134
6. Question
Which of the following pairs of ions is isoelectronic and isostructural? [NEET-II 2016]
CorrectIncorrectHint
(d) Number of electrons
\(
\begin{aligned}
&\mathrm{CO}_{3}^{2-}=6+2+24=32 \\
&\mathrm{SO}_{3}^{2-}=16+2+24=42 \\
&\mathrm{CIO}_{3}^{-}=17+24+1=42 \\
&\mathrm{CO}_{3}^{2-}=6+24+2=32 \\
&\mathrm{NO}_{3}^{2-}=7+2+24=33
\end{aligned}
\)
Hence, \(\mathrm{CIO}_{3}^{-}\)and \(\mathrm{SO}_{3}^{2-}\) are isoelectronic and are pyramidal in shape.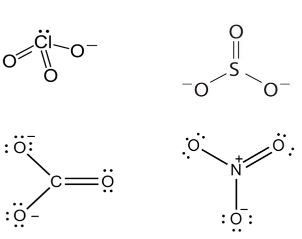
-
Question 7 of 134
7. Question
.The correct geometry and hybridization for \(\mathrm{XeF}_{4}\) are [NEET-II 2016]
CorrectIncorrectHint
(a) The correct option is ‘a’, Octahedral, \(\mathrm{sp}^{3} \mathrm{~d}^{2}\)
\(\mathrm{XeF}_{4}\) is \(\mathrm{AB}_{4} \mathrm{~L}_{2}\) type in which the xenon is bonded with four fluorine atoms with 4 sigma bonds and xenon has two lone pairs (4 sigma bonds and 2 lone pairs).
So, the hybridisation is \(\mathrm{sp}^{3} \mathrm{~d}^{2}\).
Geometry is octahedral and shape is square planar.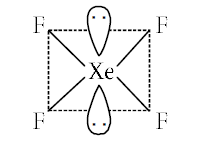
-
Question 8 of 134
8. Question
Among the following, which one is a wrong statement? [NEET-II 2016]
CorrectIncorrectHint
(c)
-
Question 9 of 134
9. Question
Consider the molecules \(\mathrm{CH}_{4}, \mathrm{NH}_{3}\) and \(\mathrm{H}_{2} \mathrm{O}\). Which of the given statements is false? [NEET-II 2016]
CorrectIncorrectHint
(d)
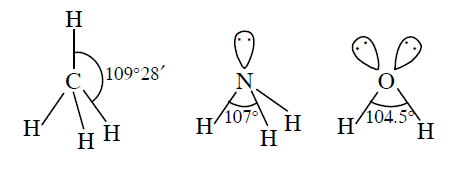
-
Question 10 of 134
10. Question
Predict the correct order among the following [NEET-I 2016]
CorrectIncorrectHint
(c) According to VSEPR theory, the repulsive forces between lone pair and lone pair are greater than between lone pair and bond pair which are further greater than bond pair and bond pair
-
Question 11 of 134
11. Question
In which of the following pairs, both the species are not isostructural? [AIPMT 2015]
CorrectIncorrectHint
(c) In diamond and silicon carbide, central atom is \(s p^{3}\) hybridised, and hence, both are isostructural. \(\mathrm{NH}_{3}\) and \(\mathrm{PH}_{3}\), both are pyramidal and central atom in both cases is
\(s p^{3}\) hybridised.
\(\mathrm{SiCl}_{4}\) and \(\mathrm{PCl}_{4}^{+}\), both are tetrahedral and central atom in both cases is \(s p^{3}\) hybridised.In \(\mathrm{XeF}_{4}\), Xe is \(s p^{3} d^{2}\) hybridised and structure is square planar while in \(\mathrm{XeO}_{4}\), Xe is \(s p^{3}\) hybridised and structure is tetrahedral.
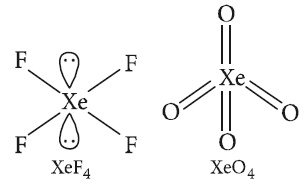
-
Question 12 of 134
12. Question
Decreasing order of stability of \(\mathrm{O}_{2}, \mathrm{O}_{2}^{-}, \mathrm{O}_{2}^{+}\) and \(\mathrm{O}_{2}^{2-}\) is [NEET 2015]
CorrectIncorrectHint
(d) \(
\begin{array}{r}
\mathrm{O}_2(16): K K \sigma 2 s^2 \sigma^* 2 s^2 \sigma 2 p_{\mathrm{z}}^2 \pi 2 p_x^2=\pi 2 p_y^2 \\
\pi^* 2 p_x{ }^1=\pi^* 2 p_y{ }^1
\end{array}
\)
\(
\text { Bond order }=\frac{1}{2}(8-4)=2
\)
\(
\begin{array}{l}
\mathrm{O}_2^{-}(17): K K \sigma 2 s^2 \sigma^* 2 s^2 \sigma 2 p_{\mathrm{z}}^2 \pi 2 p_x^2=\pi 2 p_y^2 \\
\pi^* 2 p_x^2=\pi^* 2 p_y{ }^1
\end{array}
\)
\(
\text { Bond order }=\frac{1}{2}(8-5)=1.5
\)
\(
\mathrm{O}_2^{+}(15): K K \sigma 2 s^2 \sigma^* 2 s^2 \sigma 2 p_z^2 \pi 2 p_x^2=\pi 2 p_y^2 \pi^* 2 p_x{ }^1
\)
\(
\text { Bond order }=\frac{1}{2}(8-3)=2.5
\)Bond order \(\propto\) stability. The decreasing order of stability is
\(
\mathrm{O}_{2}^{+}>\mathrm{O}_{2}>\mathrm{O}_{2}^{-}>\mathrm{O}_{2}{ }^{2-}
\) -
Question 13 of 134
13. Question
Which of the following pairs of ions are isoelectronic and isostructural? [NEET 2015]
CorrectIncorrectHint
(b) \(
\begin{array}{|l|c|c|c|}
\hline \text { Species } & \text { Hybridisation } & \text { Shape } & \begin{array}{c}
\text { No. of } \\
\boldsymbol{e}^{-} \mathbf{s}
\end{array} \\
\hline \mathrm{SO}_{3}{ }^{2-} & s p^{3} & \text { Pyramidal } & 42 \\
\hline \mathrm{ClO}_{3}{ }^{-} & s p^{3} & \text { Pyramidal } & 42 \\
\hline \mathrm{CO}_{3}{ }^{2-} & s p^{2} & \begin{array}{c}
\text { Triangular } \\
\text { planar }
\end{array} & 32 \\
\hline \mathrm{NO}_{3}^{-} & s p^{2} & \begin{array}{c}
\text { Triangular } \\
\text { planar }
\end{array} & 32 \\
\hline
\end{array}
\) -
Question 14 of 134
14. Question
The correct bond order in the following species is [NEET 2015]
CorrectIncorrectHint
(b) \(\quad \mathrm{O}_{2}^{-} \quad \mathrm{O}_{2} \quad \mathrm{O}_{2}^{+} \quad \mathrm{O}_{2}{ }^{2+}\)
B.O.\(\quad 1.5 \quad 2.0 \quad 2.5 \quad 3.0\) -
Question 15 of 134
15. Question
Which of the following options represents the correct bond order? [NEET 2015
CorrectIncorrectHint
(d) \(\quad \mathrm{O}_{2}^{-} \lt \quad \mathrm{O}_{2} \lt \quad \mathrm{O}_{2}^{+}\)
B.O.\(\quad 1.5 \quad \quad 2.0 \quad \quad 2.5\) -
Question 16 of 134
16. Question
Maximum bond angle at nitrogen is present in which of the following? [NEET 2015]
CorrectIncorrectHint
(a)
\(
\begin{array}{|l|c|l|c|c|}
\hline \text { Species } & \mathrm{NO}_{3}^{-} & \mathrm{NO}_{2} & \mathrm{NO}_{2}^{-} & \mathrm{NO}_{2}^{+} \\
\hline \text { Hybridisation } & s p^{2} & s p^{2} & s p^{2} & s p \text { (linear) } \\
\hline \text { Bond angle } & 120^{\circ} & 134^{\circ} & 115^{\circ} & 180^{\circ} \\
\hline
\end{array}
\)
So, \(\mathrm{NO}_{2}^{+}\)has maximum bond angle. -
Question 17 of 134
17. Question
Which of the following molecules has the maximum dipole moment? [NEET 2014]
CorrectIncorrectHint
(c) In \(\mathrm{NH}_{3}, \mathrm{H}\) is less electronegative than \(\mathrm{N}\) and hence dipole moment of each \(\mathrm{N}-\mathrm{H}\) bond is towards \(\mathrm{N}\) and create high net dipole moment whereas in \(\mathrm{NF}_{3}, \mathrm{~F}\) is more electronegative than \(\mathrm{N}\), the dipole moment of each \(\mathrm{N}-\mathrm{F}\) bond is opposite to that of lone pair, hence reducing the net dipole moment.
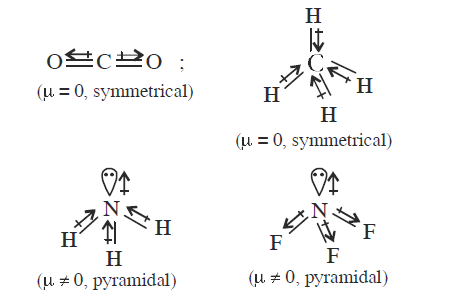
-
Question 18 of 134
18. Question
.Which one of the following species has plane triangular shape? [AIPMT 2014]
CorrectIncorrectHint
(b)
-
Question 19 of 134
19. Question
Which of the following is electron-deficient? [NEET 2013]
CorrectIncorrectHint
(a) Boron hydrides are electron deficient compound
-
Question 20 of 134
20. Question
\(\mathrm{XeF}_{2}\) is isostructural with [NEET 2013]
CorrectIncorrectHint
(d)
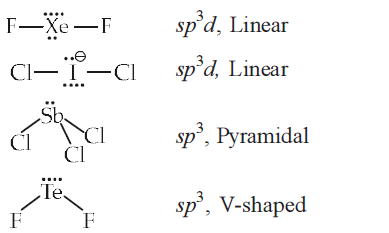
-
Question 21 of 134
21. Question
Which of the following is a polar molecule? [NEET 2013]
CorrectIncorrectHint
(d) \(\mathrm{SF}_{4}\) has \(s p^{3} d\)-hybridisation and see-saw shape with \((4 b p+1 l p)\)

-
Question 22 of 134
22. Question
Which of the following is paramagnetic? [NEET 2013]
CorrectIncorrectHint
(d) \(\mathrm{O}_{2}^{-}\)(17) superoxide has one unpaired electron.
\(
\begin{aligned}
\sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \sigma 2 p_{z}^{2} \pi 2 p_{x}^{2}=& \pi 2 p_{y}^{2} \\
& \pi^{*} 2 p_{x}^{2}=\pi^{*} 2 p_{y}^{1}
\end{aligned}
\) -
Question 23 of 134
23. Question
Dipole-induced dipole interactions are present in which of the following pairs [NEET 2013]
CorrectIncorrectHint
(a) \(\mathrm{HCl}\) is polar \((\mu \neq 0)\) and \(\mathrm{He}\) is non-polar \((\mu=0)\) gives dipole-induced dipole interaction
-
Question 24 of 134
24. Question
The pair of species that has the same bond order in the following is [NEET Karnatak 2013]
CorrectIncorrectHint
(a) \(\mathrm{CO}=6+8=14\) electrons, \(\mathrm{NO}^{+}=7+8-1=14\) electrons
Both have electronic configuration : \(\sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \sigma 2 p_{z}^{2} \pi 2 p_{x}^{2} \pi 2 p_{y}^{2}\)
So both have bond order \(=\frac{10-4}{2}=3\) -
Question 25 of 134
25. Question
The outer orbitals of \(\mathrm{C}\) in ethene molecule can be considered to be hybridized to give three equivalent \(s p^{2}\) orbitals. The total number of sigma \((\sigma)\) and pi \((\pi)\) bonds in ethene molecule is [NEET Karnatak 2013]
CorrectIncorrectHint
(c)
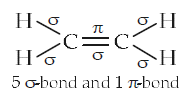
-
Question 26 of 134
26. Question
In which of the following pair both the species have \(s p^{3}\) hybridization? [NEET Karnatak 2013]
CorrectIncorrectHint
\({ (b) } \mathrm{NF}_{3} \text { and } \mathrm{H}_{2} \mathrm{O} \text { are } s p^{3} \text {-hybridisation. }\)
-
Question 27 of 134
27. Question
In which of the following ionization processes the bond energy increases and the magnetic behaviour changes from paramagnetic to diamagnetic. [NEET Karnatak 2013]
CorrectIncorrectHint
(c) In \(C_{2}-C_{2}^{+}\)electron is removed from bonding molecular orbital so bond order decreases. In \(\mathrm{NO} \longrightarrow \mathrm{NO}^{+}\), electron is removed from anti bonding molecular orbital so bond order increases and nature changes from paramagnetic to diamagnetic.
Molecular orbital configuration of \(\mathrm{O}_{2} \Rightarrow \sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \sigma 2 p_{z}{ }^{2} \pi 2 p_{x}{ }^{2} \pi 2 p_{y}{ }^{2} \pi^{*} 2 p_{x}{ }^{1}\) \(\pi^{*} 2 p_{y}{ }^{1}\)
\(\Rightarrow\) Paramagnetic
Bond order \(=\frac{10-6}{2}=2\)
\(\mathrm{O}_{2}^{+} \Rightarrow \sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \sigma 2 p_{z}{ }^{2} \pi 2 p_{x}{ }^{2} \pi 2 p_{y}{ }^{2} \pi^{*} 2 p_{x}{ }^{1}\)
\(\Rightarrow\) Paramagnetic
Bond order \(=\frac{10-5}{2}=2.5\)
\(\mathrm{C}_{2} \Rightarrow \sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \pi 2 p_{x}^{2} \pi 2 p_{y}^{2}\)
\(\Rightarrow\) Diamagnetic
Bond order \(=\frac{8-4}{2}=2\)
\(\mathrm{C}_{2}^{+} \Rightarrow \sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \pi 2 p_{x}{ }^{2} \pi 2 p_{y}{ }^{1}\)
\(\Rightarrow\) Paramagnetic
Bond order \(=\frac{7-4}{2}=1.5\)
\(\mathrm{NO} \Rightarrow \sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \sigma 2 p_{z}{ }^{2} \pi 2 p_{x}{ }^{2} \pi 2 p_{y}{ }^{2} \pi^{*} 2 p_{x}{ }^{1}\)
\(\Rightarrow\) Paramagnetic
Bond order \(=\frac{10-5}{2}=2.5\)
\(\mathrm{NO}^{+} \Rightarrow \sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \sigma 2 p_{z}{ }^{2} \pi 2 p_{x}{ }^{2} \pi 2 p_{y}{ }^{2}\)
\(\Rightarrow\) Diamagnetic.
Bond order \(=\frac{10-4}{2}=3\)
\(\mathrm{N}_{2} \Rightarrow \sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \pi 2 p_{x}^{2} \pi 2 p_{y}^{2} \sigma 2 p_{z}^{2}\)
\(\Rightarrow\) Paramagnetic
Bond order \(=\frac{10-4}{2}=3\)
\(\mathrm{N}_{2}^{+} \Rightarrow \sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \pi 2 p_{x}{ }^{2} \pi 2 p_{y}{ }^{2} \sigma 2 p_{z}{ }^{1}\)
\(\Rightarrow\) Paramagnetic
Bond order \(=\frac{9-4}{2}=2.5\). -
Question 28 of 134
28. Question
Which one of the following pairs is isostructural (i.e., having the same shape and hybridization)? [AIPMT 2012]
CorrectIncorrectHint
(d)
\(\begin{aligned}
\mathrm{BCl}_{3} \Rightarrow s p^{2}, & \text { trigonal planar } \\
\mathrm{BrCl}_{3} \Rightarrow s p^{3} d, & \text { T-shaped } \\
\mathrm{NH}_{3} \Rightarrow s p^{3}, & \text { pyramidal } \\
\mathrm{NO}_{3}^{-} \Rightarrow s p^{2}, & \text { trigonal planar } \\
\mathrm{NF}_{3} \Rightarrow s p^{3}, & \text { pyramidal } \\
\mathrm{BF}_{3} \Rightarrow s p^{2}, & \text { trigonal planar } \\
\mathrm{BF}_{4}^{-} \Rightarrow s p^{3}, & \text { tetrahedral } \\
\mathrm{NH}_{4}^{+} \Rightarrow s p^{3}, & \text { tetrahedral }
\end{aligned}\) -
Question 29 of 134
29. Question
Bond order of 1.5 is shown by [AIPMT 2012]
CorrectIncorrectHint
(b) Configuration of \(\mathrm{O}_{2}\)
\(\sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \sigma 2 p_{z}^{2} \pi 2 p_{x}{ }^{2} \pi 2 p_{y}{ }^{2} \pi^{*} 2 p_{x}{ }^{1} \pi^{*} 2 p_{y}{ }^{1}\)
No. of \(e^{-}\)in No. of \(e^{-}\)in
Bond order \(=\frac{\text { bonding M.O. } – \quad \text { antibonding M.O. }}{2}\)
Bond order of \(\mathrm{O}_{2}^{+}=\frac{10-5}{2}=2.5\)
Bond order of \(\mathrm{O}_{2}^{-}=\frac{10-7}{2}=1.5\)
Bond order of \(\mathrm{O}_{2}^{2-}=\frac{10-8}{2}=1.0\)
Bond order of \(\mathrm{O}_{2}=\frac{10-6}{2}=2\) -
Question 30 of 134
30. Question
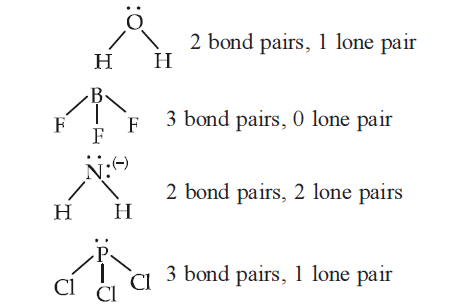
Which of the following species contains three bond pairs and one lone pair around the central atom? [AIPMT 2012]
CorrectIncorrectHint
(d)
-
Question 31 of 134
31. Question
The pair of species with the same bond order is [Mains 2012]
CorrectIncorrectHint
\(
\begin{aligned}
&\text { (a) } \mathrm{O}_{2}^{2-} \rightarrow 1 \quad \mathrm{~B}_{2} \rightarrow 1\\
&\mathrm{O}_{2}^{+} \rightarrow 2.5 \quad \mathrm{NO}^{+} \rightarrow 3\\
&\mathrm{NO} \rightarrow 2.5 \quad \mathrm{CO} \rightarrow 3\\
&\mathrm{N}_{2} \rightarrow 3 \quad \mathrm{O}_{2} \rightarrow 2.0
\end{aligned}
\) -
Question 32 of 134
32. Question
During change of \(\mathrm{O}_{2}\) to \(\mathrm{O}_{2}^{-}\)ion, the electron adds on which one of the following orbitals? [Mains 2012]
CorrectIncorrectHint
(a) Electronic configuration of \(\mathrm{O}_{2}\)
\(\sigma(1 s)^{2} \sigma^{*}(1 s)^{2} \sigma(2 s)^{2} \sigma^{*}(2 s)^{2} \sigma\left(2 p_{z}\right)^{2} \pi\left(2 p_{x}\right)^{2} \pi\left(2 p_{y}\right)^{2}\) \(\pi^{*}\left(2 p_{x}\right)^{1} \pi^{*}\left(2 p_{y}\right)^{1}\)Thus the incoming electron will enter in \(\pi^{*} 2 p_{x}\) to form \(\mathrm{O}_{2}^{-}\).
-
Question 33 of 134
33. Question
Four diatomic species are listed below. Identify the correct order in which the bond order is increasing in them [Mains 2012, 2008]
CorrectIncorrectHint
(d)
\(
\begin{array}{|c|c|}
\hline \text { Diatomic species } & \text { Bond order } \\
\hline \mathrm{NO} & 2.5 \\
\mathrm{O}_{2}{ }^{-} & 1.5 \\
\mathrm{C}_{2}{ }^{2-} & 3.0 \\
\mathrm{He}_{2}{ }^{+} & 0.5 \\
\hline
\end{array}
\)\(
\text { Thus increasing order : } \mathrm{He}_{2}^{+}<\mathrm{O}_{2}^{-}<\mathrm{NO}<\mathrm{C}_{2}^{2-}
\) -
Question 34 of 134
34. Question
Which of the following has the minimum bond length? [AIPMT 2011]
CorrectIncorrectHint
(a) Electronic configuration \(\mathrm{O}_{2}: K K(\sigma 2 s)^{2}\left(\sigma^{*} 2 s\right)^{2}\left(\sigma 2 p_{x}\right)^{2}\left(\pi 2 p_{y}\right)^{2}\left(\pi 2 p_{z}\right)^{2}\left(\pi^{*} 2 p_{y}\right)^{1}\)
\(\left(\pi^{*} 2 p_{z}\right)^{1}\)
Bond order \(=\frac{1}{2}(8-4)=2\)
\(\mathrm{O}_{2}^{+}:\)Bond order \(=\frac{1}{2}(8-3)=2 \frac{1}{2}\)
\(\mathrm{O}_{2}^{-}:\)Bond order \(=\frac{1}{2}(8-5)=1 \frac{1}{2}\)
\(\mathrm{O}_{2}^{2-}:\) Bond order \(=\frac{1}{2}(8-6)=1\)
As bond order increases, bond length decreases. -
Question 35 of 134
35. Question
Which of the two ions from the list given below that have the geometry that is explained by the same hybridization of orbitals, \(\mathrm{NO}_{2}^{-}, \mathrm{NO}_{3}^{-}\), \(\mathrm{NH}_{2}^{-}, \mathrm{NH}_{4}^{+}, \mathrm{SCN}^{-}\)? [AIPMT 2011]
CorrectIncorrectHint
(a)
\(
\begin{array}{lc}
\text { Ions } & \text { Hybridisation } \\
\mathrm{NO}_{2}^{-} & s p^{2} \\
\mathrm{NO}_{3}^{-} & s p^{2} \\
\mathrm{NH}_{2}^{-} & s p^{3} \\
\mathrm{NH}_{4}^{+} & s p^{3} \\
\mathrm{SCN}^{-} & s p
\end{array}
\) -
Question 36 of 134
36. Question
The correct order of increasing bond length of \(\mathrm{C}-\mathrm{H}, \mathrm{C}-\mathrm{O}, \mathrm{C}-\mathrm{C}\) and \(\mathrm{C}=\mathrm{C}\) is [AIPMT 2011]
CorrectIncorrectHint
(a) Increasing order of bond length is
\(
\underset{107 \mathrm{pm}}{\mathrm{C}-\mathrm{H}}<\underset{134 \mathrm{pm}}{\mathrm{C}} {\mathrm{=}}{\mathrm{C}}<\underset{141 \mathrm{pm}}{\mathrm{C}-\mathrm{O}}<\underset{154 \mathrm{pm}}{\mathrm{C}-\mathrm{C}}
\) -
Question 37 of 134
37. Question
Which of the following structures is the most preferred and hence of lowest energy for \(\mathrm{SO}_{3}\)? [Mains 2011]
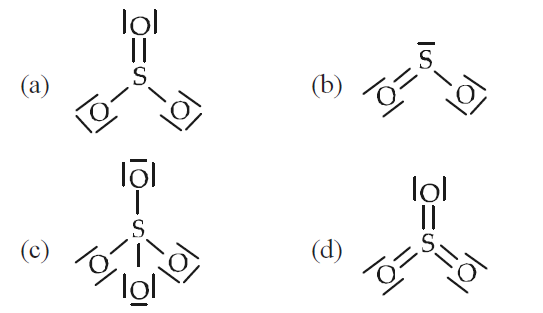 CorrectIncorrect
CorrectIncorrectHint
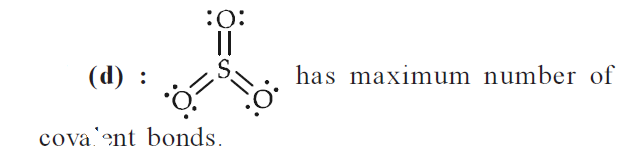
-
Question 38 of 134
38. Question
The pairs of species of oxygen and their magnetic behaviour are noted below. Which of the following presents the correct description? [2011]
CorrectIncorrectHint
(c) \(\mathrm{O}_{2}^{+}\)and \(\mathrm{O}_{2}\) are paramagnetic in nature as they contain one and two unpaired electrons resnectively
-
Question 39 of 134
39. Question
In which of the following pairs of molecules/ ions, the central atoms have \(s p^{2}\) hybridisation? [AIPMT 2010]
CorrectIncorrectHint
(b) The hybridisation of the central atom can be calculated as
\(
\mathrm{H}=\frac{1}{2}\left[\begin{array}{c}
\left.\begin{array}{l}
\text { No. of electrons } \\
\text { in valence shell } \\
\text { of atom }
\end{array}\right)+\left(\begin{array}{l}
\text { No. of monovalent } \\
\text { atoms around } \\
\text { central atom }
\end{array}\right) \\
-\left(\begin{array}{l}
\text { Charge on } \\
\text { cation }
\end{array}\right)+\left(\begin{array}{l}
\text { Charge on } \\
\text { anion }
\end{array}\right)
\end{array}\right]
\)
\(\begin{aligned}
\therefore \quad \text { For } \mathrm{BF}_{3}, \mathrm{H} &=\frac{1}{2}[(3)+(3)-(0)+(0)] \\
&=3 \Rightarrow s p^{2} \text { hybridisation. } \\
\text { For } \mathrm{NO}_{2}^{-}, \mathrm{H} &=\frac{1}{2}[(5)+(0)-(0)+(1)] \\
&=3 \Rightarrow s p^{2} \text { hybridisation. }
\end{aligned}
\) -
Question 40 of 134
40. Question
Which one of the following species does not exist under normal conditions? [AIPMT 2010]
CorrectIncorrectHint
(b) \(\mathrm{Be}_{2}\) does not exist.
\(\mathrm{Be}_{2}\) has an electronic configuration of :
\(
\sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2}
\)
\(\therefore \quad\) Bond order \(=\frac{4-4}{2}=0\)
Thus, \(\mathrm{Be}_{2}\) does not exist. -
Question 41 of 134
41. Question
In which one of the following species the central atom has the type of hybridization which is not the same as that present in the other three? [2010]
CorrectIncorrectHint
(c) Hybridisation of the central atom can be calculated as:
\(
\mathrm{H}=\frac{1}{2}\left[\begin{array}{c}
\left(\begin{array}{l}
\text { No. of valence } \\
\text { electrons in the } \\
\text { central atom }
\end{array}\right)+\left(\begin{array}{l}
\text { No. of monovalent } \\
\text { atoms around } \\
\text { central atom }
\end{array}\right) \\
-\left(\begin{array}{l}
\text { Charge on } \\
\text { cation }
\end{array}\right)+\left(\begin{array}{l}
\text { Charge on } \\
\text { anion }
\end{array}\right)
\end{array}\right]
\)
Applying this formula we find that all the given species except \(\left[\mathrm{SbCl}_{5}\right]^{2-}\) have central atom with \(s p^{3} d\) (corresponding to \(\mathrm{H}=5\) ) hybridization. In \(\left[\mathrm{SbCl}_{5}\right]^{2-}\), Sb is \(s p^{3} d^{2}\) hybridized. -
Question 42 of 134
42. Question
In which of the following molecules the central atom does not have \(s p^{3}\) hybridization? [Mains 2010]
CorrectIncorrectHint
(b) For neutral molecules,
No. of electron pairs \(=\) No. of atoms bonded to it \(+\) \(1 / 2\) [Gp. no. of central atom – Valency of central atom]
\(\therefore \quad\) For \(\mathrm{CH}_{4}\), no. of \(e^{-}\)pairs \(=4+\frac{1}{2}[4-4]\) \(=4\left(s p^{3}\right.\) hybridisation)For \(\mathrm{SF}_{4}\), no. of \(e^{-}\)pairs \(=4+\frac{1}{2}[6-4]\) \(=5\left(s p^{3} d\right.\) hybridisation \()\)
For ions,
No. of electron pairs \(=\) No. of atoms bonded to it \(+\) \(1 / 2\) [Gp. no. of central atom – Valency of central atom \(\pm\) No. of electrons]
\(
\begin{aligned}
&\therefore \quad \text { For } \mathrm{BF}_{4}^{-}, \text {no. of } e^{-} \text {pairs }=4+\frac{1}{2}[3-4+1] \\
&=4\left(s p^{3} \text { hybridisation }\right) \\
&\text { For } \mathrm{NH}_{4}^{+}, \text {no. of } e^{-} \text {pairs }=4+\frac{1}{2}[5-4-1] \\
&=4\left(s p^{3} \text { hybridisation }\right)
\end{aligned}
\) -
Question 43 of 134
43. Question
Some of the properties of the two species, \(\mathrm{NO}_{3}^{-}\)and \(\mathrm{H}_{3} \mathrm{O}^{+}\)are described below. Which one of them is correct? [Mains 2010]
CorrectIncorrectHint
(a) No. of electron pairs at the central atom \(=\) No. of atoms bonded to it \(+1 / 2\) [Group number of central atom – Valency of the central atom \(\pm\) no. of electrons]
No. of electron pairs at the central atom in \(\mathrm{NO}_{3}^{-}\) \(=3+\frac{1}{2}[5-6+1]=3 \quad\left(s p^{2}\right.\) hybridisation \()\).
No. of electron pairs at the central atom in \(\mathrm{H}_{3} \mathrm{O}^{+}=3+\frac{1}{2}[6-3-1]=4 \quad\left(s p^{3}\right.\) hybridisation \()\). -
Question 44 of 134
44. Question
What is the dominant intermolecular force or bond that must be overcome in converting liquid \(\mathrm{CH}_{3} \mathrm{OH}\) to a gas? [AIPMT 2009]
CorrectIncorrectHint
(d) Methanol can undergo intermolecular association through \(\mathrm{H}\)-bonding as the \(-\mathrm{OH}\) group in alcohols is highly polarised.
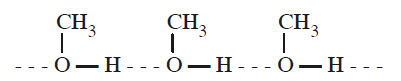
As a result, in order to convert liquid \(\mathrm{CH}_{3} \mathrm{OH}\) to gaseous state, the strong hydrogen bonds must be broken.
-
Question 45 of 134
45. Question
According to \(\mathrm{MO}\) theory which of the lists ranks the nitrogen species in terms of increasing bond order? [AIPMT 2009]
CorrectIncorrectHint
(a) According to MOT, the molecular orbital electronic configuration of
\(\mathrm{N}_{2}:(\sigma 1 s)^{2}\left(\sigma^{*} 1 s\right)^{2}(\sigma 2 s)^{2}\left(\sigma^{*} 2 s\right)^{2}\left(\pi 2 p_{x}\right)^{2}\left(\pi 2 p_{y}\right)^{2}\left(\sigma 2 p_{z}\right)^{2}\)
\(
\begin{aligned}
\therefore \text { B.O }=\frac{10-4}{2}=3 \\
\mathrm{~N}_{2}^{-}:(\sigma 1 s)^{2}\left(\sigma^{*} 1 s\right)^{2}(\sigma 2 s)^{2}\left(\sigma^{*} 2 s\right)^{2}\left(\pi 2 p_{x}\right)^{2}\left(\pi p_{y}\right)^{2} \\
\quad\left(\sigma 2 p_{z}\right)^{2}\left(\pi^{*} 2 p_{x}\right)^{1}
\end{aligned}
\)
\(
\begin{aligned}
&\therefore \quad \text { B.O }=\frac{10-5}{2}=2.5 \\
&\mathrm{~N}_{2}^{2-}:(\sigma 1 s)^{2}\left(\sigma^{*} 1 s\right)^{2}(\sigma 2 s)^{2}\left(\sigma^{*} 2 s\right)^{2}\left(\pi 2 p_{x}\right)^{2}\left(\pi 2 p_{y}\right)^{2} \\
&\therefore \quad\left(\sigma 2 p_{z}\right)^{2}\left(\pi^{*} 2 p_{x}\right)^{1}\left(\pi^{*} 2 p_{y}\right)^{1} \\
&\therefore \quad \text { B.O. }=\frac{10-6}{2}=2 .
\end{aligned}
\)
Hence the order : \(\mathrm{N}_{2}^{2-}<\mathrm{N}_{2}^{-}<\mathrm{N}_{2}\) -
Question 46 of 134
46. Question
In which of the following molecules/ions \(\mathrm{BF}_{3}\), \(\mathrm{NO}_{2}^{-}, \mathrm{NH}_{2}^{-}\)and \(\mathrm{H}_{2} \mathrm{O}\), the central atom is \(s p^{2}\) hybridised? [AIPMT 2009]
CorrectIncorrectHint
(c) \(\mathrm{BF}_{3} \rightarrow s p^{2}, \mathrm{NO}_{2}^{-} \rightarrow s p^{2}, \mathrm{NH}_{2}^{-} \rightarrow s p^{3}\),
\(
\mathrm{H}_{2} \mathrm{O} \rightarrow s p^{3}
\) -
Question 47 of 134
47. Question
The correct order of increasing bond angles in the following triatomic species is [AIPMT 2008]
CorrectIncorrectHint
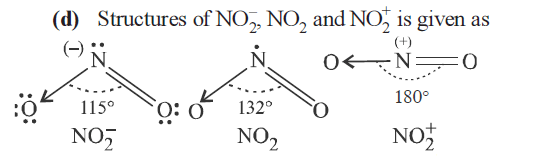
The correct order of increasing bond angles in the following triatomic species is
\(
\mathrm{NO}_{2}^{-}<\mathrm{NO}_{2}>\mathrm{NO}_{2}^{+}
\) -
Question 48 of 134
48. Question
In which of the following pairs, the two species are isostructural? [AIPMT 2007]
CorrectIncorrectHint
(c) Hybridisation of \(\mathrm{Br}\) in \(\mathrm{BrO}_{3}^{-}\): \(H=1 / 2(7+0-0+1)=4\) i.e.sp \(p^{3}\) hybridisation Hybridisation of \(\mathrm{Xe}\) in \(\mathrm{XeO}_{3}\) : \(H=\frac{1}{2}(8+0-0+0)=4\) i.e. \(s p^{3}\) hybridisation In both \(\mathrm{BrO}_{3}^{-}\)and \(\mathrm{XeO}_{3}\), the central atom is \(s p^{3}\) hybridised and contains one lone pair of electrons, hence in both the cases, the structure is trigonal pyramidal.
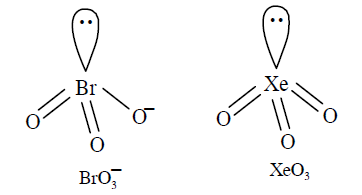
-
Question 49 of 134
49. Question
The correct order of \(\mathrm{C}-\mathrm{O}\) bond length among \(\mathrm{CO}, \mathrm{CO}_{3}{ }^{2-}, \mathrm{CO}_{2}\) is [AIPMT 2007]
CorrectIncorrectHint
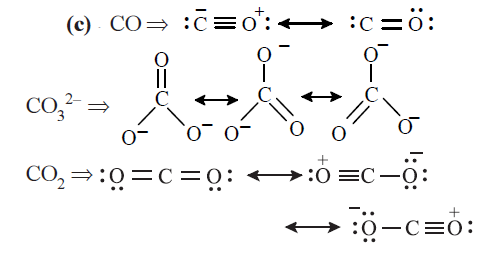
More single bond character in resonance hybrid, more is the bond length. Hence the increasing bond length is
\(
\mathrm{CO}<\mathrm{CO}_{2}<\mathrm{CO}_{3}{ }^{2-}
\) -
Question 50 of 134
50. Question
Which of the following is not a correct statement? [AIPMT 2006]
CorrectIncorrectHint
(d) For \(A B_{5}\) molecules, there are three possible geometries i.e. planar pentagonal, square pyramidal, and trigonal bipyramidal
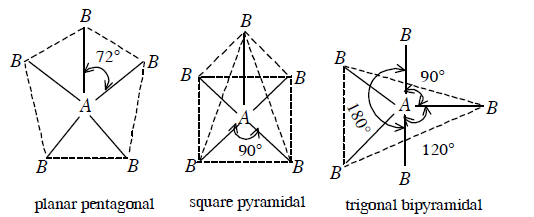
Out of these three geometries, it is only trigonal pyramidal shape in which bond pair-bond pair repulsions are minimum and hence this geometry is the most probable geometry of \(A B_{5}\) molecule.
-
Question 51 of 134
51. Question
Which of the following species has a linear shape? [AIPMT 2006]
CorrectIncorrectHint
-
Question 52 of 134
52. Question
Which of the following is not isostructural with \(\mathrm{SiCl}_{4}\)? [AIPMT 2006]
CorrectIncorrectHint
(b) \(\mathrm{SiCl}_{4}, \mathrm{NH}_{4}^{+}, \mathrm{SO}_{4}^{2-}\) and \(\mathrm{PO}_{4}^{3-}\) ions are the examples of molecules/ions which are of \(A B_{4}\) type and have tetrahedral structure. \(\mathrm{SCl}_{4}\) is \(\mathrm{AB}_{4}\) (lone pair) types species. Although the arrangement of five \(s p^{3} d\) hybrid orbitals in space is trigonal bipyramidal, due to the presence of one lone pair of electron in the basal hybrid orbital, the shape of \(A B_{4}\) (lone pair) species gets distorted and becomes distorted tetrahedral or see-saw.
-
Question 53 of 134
53. Question
Which of the following molecules has trigonal planar geometry? [AIPMT 2005]
CorrectIncorrectHint
-
Question 54 of 134
54. Question
The correct order in which the \(\mathrm{O}-\mathrm{O}\) bond length increases in the following is [AIPMT 2005]
CorrectIncorrectHint
(d) Bond lengths of \(\mathrm{O}-\mathrm{O}\) in \(\mathrm{O}_{2}\) is 1.21 Å, in \(\mathrm{H}_{2} \mathrm{O}_{2}\) is 1.48 Å and in \(\mathrm{O}_{3}\) is 1.28 Å. Therefore, correct order of the \(\mathrm{O}-\mathrm{O}\) bond length is \(\mathrm{H}_{2} \mathrm{O}_{2}>\mathrm{O}_{3}>\mathrm{O}_{2}\).
-
Question 55 of 134
55. Question
The surface tension of which of the following liquid is maximum? [AIPMT 2005]
CorrectIncorrectHint
(c) Hydrogen bonding in \(\mathrm{H}_{2} \mathrm{O}>\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\)
\(>\mathrm{CH}_{3} \mathrm{OH}\)
Hence, \(\mathrm{H}_{2} \mathrm{O}\) has maximum surface tension. -
Question 56 of 134
56. Question
Among the following, the pair in which the two species are not isostructural is [AIPMT 2004]
CorrectIncorrectHint
(a) \(\mathrm{SiF}_{4}\) has symmetrical tetrahedral shape which is due to \(s p^{3}\) hybridisation of the central silicon atom in its excited state configuration. \(\mathrm{SF}_{4}\) has distorted tetrahedral or sea-saw geometry which arises due to \(s p^{3} d\) hybridisation of central sulphur atom and due to the presence of one lone pair of electrons in one of the equatorial hybrid orbital.
-
Question 57 of 134
57. Question
In a regular octahedral molecule, \(M X_{6}\) the number of \(X-M-X\) bonds at \(180^{\circ}\) is [AIPMT 2004]
CorrectIncorrectHint
(a) In octahedral molecule six hybrid orbitals directed towards the corners of a regular octahedron with a bond angle of \(90^{\circ}\).
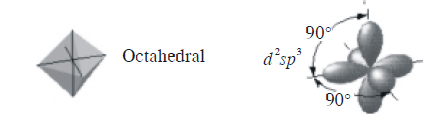
According to this geometry, the number of \(X-M-X\) bonds at \(180^{\circ}\) must be three.
-
Question 58 of 134
58. Question
\(\mathrm{H}_{2} \mathrm{O}\) is dipolar, whereas \(\mathrm{BeF}_{2}\) is not. It is because [AIPMT 2004]
CorrectIncorrectHint
(d) The overall value of the dipole moment of a polar molecule depends on its geometry and shape, i.e. vectorial addition of dipole moment of the constituent bonds. Water has angular structure with bond angle \(105^{\circ}\) as it has dipole moment. However \(\mathrm{BeF}_{2}\) is a linear molecule since dipole moment summation of all the bonds present in the molecule cancel each other.
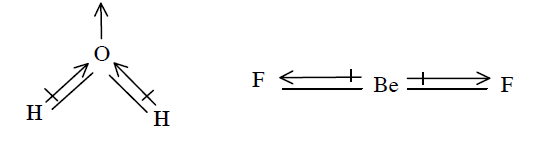
-
Question 59 of 134
59. Question
In \(\mathrm{BrF}_{3}\) molecule, the lone pairs occupy equatorial positions to minimize AIPMT 2004]
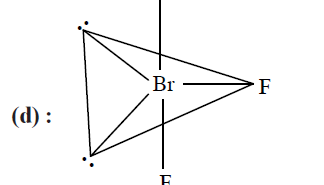
CorrectIncorrectHint
Bent T-shaped geometry in which both lone pairs occupy the equatorial positions of the trigonal bipyramid. Here \((l p-l p)\) repulsion \(=0,(l p-b p)\) repulsion \(=4\) and \((b p-b p)\) repulsion \(=2\).
-
Question 60 of 134
60. Question
Which one of the following statements is not correct for sigma- and pi- bonds formed between two carbon atoms? [AIPMT 2003]
CorrectIncorrectHint
(b) We know C – C \(=347 \mathrm{~kJ} / \mathrm{mol}\) \(\mathrm{C}=\mathrm{C}=619 \mathrm{~kJ} / \mathrm{mol}\)
-
Question 61 of 134
61. Question
Which of the following has \(p \pi-d \pi\) bonding? [AIPMT 2002]
CorrectIncorrectHint
-
Question 62 of 134
62. Question
In \(\mathrm{NO}_{3}{ }^{-}\)ion number of bond pair and lone pair of electrons on nitrogen atom are [AIPMT 2002]
CorrectIncorrectHint
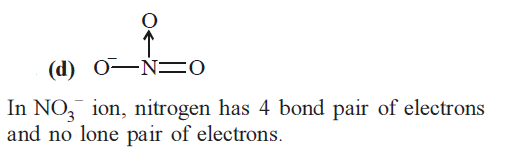
-
Question 63 of 134
63. Question
Which of the following is isoelectronic? [AIPMT 2002]
CorrectIncorrectHint
(c) In \(\mathrm{CO}\), the number of electrons \(=6+8=14[\mathrm{Z}\) of \(\mathrm{C}=6\) and \(\mathrm{O}=8]\)
Electronic configuration of molecular orbital of \(\mathrm{CO}\) : \((\sigma 1 s)^{2}\left(\sigma^{*} 1 s\right)^{2}(\sigma 2 s)^{2}\left(\sigma^{*} 2 s\right)^{2}\left(\pi 2 p_{x}\right)^{2}\left(\pi 2 p_{y}\right)^{2}\left(\sigma 2 p_{z}\right)^{2}\) \(\mathrm{CN}^{-}\)have also get \((6+7+1) 14\) electrons and the configuration is similar to that of \(\mathrm{CO}\). \(\mathrm{CN}^{-}\)and \(\mathrm{CO}\) are isoelectronic. -
Question 64 of 134
64. Question
Which of the following two are isostructural? [AIPMT 2001]
CorrectIncorrectHint
(a) Compounds having same shape with same hybridisation are known as isostructural.
\(\mathrm{XeF}_{2}, \mathrm{IF}_{2}^{-} \rightarrow\) both are \(s p^{3} d\) hybridised linear molecules. -
Question 65 of 134
65. Question
In which of the following bond angle is maximum? [AIPMT 2001]
CorrectIncorrectHint
(b) Bond angle is maximum in \(\mathrm{NH}_{4}^{+}\)tetrahedral molecule with bond angle \(109^{\circ}\).
-
Question 66 of 134
66. Question
Nitrogen forms \(\mathrm{N}_{2}\), but phosphorus does not form \(\mathrm{P}_{2}\), however, it converts \(\mathrm{P}_{4}\), reason is [CBSE AIPMT 2001]
CorrectIncorrectHint
(b) For strong \(\pi\)-bonding, \(p \pi-p \pi\) bonding should be strong. In case of \(\mathrm{P}\), due to larger size as compared to \(\mathrm{N}\)-atom, \(p \pi-p \pi\) bonding is not so strong.
-
Question 67 of 134
67. Question
\(\operatorname{In} X-\mathrm{H}—Y, X\) and \(Y\) both are electronegative elements. Then [AIPMT 2001]
CorrectIncorrectHint
(a) In X-H–Y, ‘ \(\mathrm{H}\) ‘ is directly bonded to ‘ \(\mathrm{X}\) ‘ and there is a hydrogen bond between ‘ \(\mathrm{H}\) ‘ and ‘ \(\mathrm{Y}\) ‘. As ‘ \(\mathrm{X}\) ‘ and ” \(\mathrm{Y}\) ‘ are electronegative elements that pull electron density towards themselves. As ‘ \(\mathrm{X}\) ‘ is directly bonded, it pulls more electron density. So electron density on \(\mathrm{X}\) increases on ‘ \(\mathrm{H}\) ‘ electron density decreases.
-
Question 68 of 134
68. Question
\(d \pi-p \pi\) bond present in [AIPMT 2000]
CorrectIncorrectHint
(b) In \(\mathrm{PO}_{4}^{3-}, \mathrm{P}\) atom has vacant \(d\)-orbitals, thus it can form \(p \pi-d \pi\) bond. ‘ \(\mathrm{N}\) ‘ and ‘ \(\mathrm{C}\) ‘ have no vacant ‘ \(d\) ‘ orbital in their valence shell, so they cannot form such bond.
-
Question 69 of 134
69. Question
Right order of dissociation energy \(\mathrm{N}_{2}\) and \(\mathrm{N}_{2}\) is [AIPMT 2000]
CorrectIncorrectHint
(a) \(\mathrm{N}_{2}(14) \rightarrow(\sigma 1 s)^{2},\left(\sigma^{*} 1 s\right)^{2},(\sigma 2 s)^{2},\left(\sigma^{*} 2 s\right)^{2}\)
\(
\left(\sigma^{*} 2 s\right)^{2},\left(\pi 2 p_{x}\right)^{2},\left(\pi 2 p_{y}\right)^{2},\left(\sigma 2 p_{z}\right)^{2}
\)In \(\mathrm{N}_{2}\), bond order \(=\frac{N_{b}-N_{a}}{2}=\frac{10-4}{2}=3\)
In \(\mathrm{N}_{2}^{+}\), bond order \(=\frac{9-4}{2}=2 \cdot 5\)
As the bond order in \(\mathrm{N}_{2}\) is more than \(\mathrm{N}_{2}^{+}\)so the dissociation energy of \(\mathrm{N}_{2}\) is higher than \(\mathrm{N}_{2}^{+}\). -
Question 70 of 134
70. Question
Which species does not exhibit paramagnetism? [AIPMT 2000]
CorrectIncorrectHint
(c) In ‘ \(\mathrm{CO}\) ‘ (14 electrons), there is no unpaired electron in its molecular orbital. Therefore this does not exhibit paramagnetism.
-
Question 71 of 134
71. Question
The number of anti-bonding electron pairs in \(\mathrm{O}_{2}^{2-}\) molecular ion on the basis of molecular orbital theory is (Atomic number of \(\mathrm{O}\) is 8) [AIPMT 1998]
CorrectIncorrectHint
(d) \(\mathrm{O}_{2}^{2-}(18) \rightarrow(\sigma 1 s)^{2},(\sigma * 1 s)^{2}(\sigma 2 s)^{2},\left(\sigma^{*} 2 s\right)^{2}\) \(\left(\sigma 2 p_{x}\right)^{2},\left(\pi 2 p_{y}\right)^{2},\left(\pi 2 p_{z}\right)^{2},\left(\pi * 2 p_{y}\right)^{2},\left(\pi^{*} 2 p_{z}\right)^{2}\) \(\rightarrow\) represents antibonding molecular orbitals.
Thus the no. of antibonding electrons in \(\mathrm{O}_{2}^{2-}\) ion is \(=8(4\) pairs \()\)
-
Question 72 of 134
72. Question
In \(\mathrm{PO}_{4}{ }^{3-}\) ion, the formal charge on each oxygen atom and \(\mathrm{P}-\mathrm{O}\) bond order respectively are [AIPMT 1998]
CorrectIncorrectHint
(a) The total charge \(=-3\)
So the average formal charge on each ‘ \(\mathrm{O}\) ‘ atom is \(-3 / 4=-0.75\)Again total no. of electrons in the valence shell of \(\mathrm{PO}_{4}^{3-}\) ion \(=5+8=13\)
No. of electrons involved in bond formation in \(\mathrm{PO}_{4}^{3-}\) ion \(=13-3=10\)
No. of bonds in \(\mathrm{PO}_{4}^{3-}=\frac{10}{2}=5\)
\(\Rightarrow\) Average \(\mathrm{P}-\mathrm{O}\) bond order \(=\frac{5}{4}=1.25\) -
Question 73 of 134
73. Question
\(\mathrm{N}_{2}\) and \(\mathrm{O}_{2}\) are converted into monocations, \(\mathrm{N}_{2}^{+}\)and \(\mathrm{O}_{2}^{+}\)respectively. Which is wrong? [AIPMT 1997]
CorrectIncorrectHint
(b) Diamagnetism is caused due to the absence of unpaired electrons. But in \(\mathrm{N}^{2+}\), there is unpaired electron. So, it is paramagnetic.
-
Question 74 of 134
74. Question
\(\mathrm{N}_{2}\) and \(\mathrm{O}_{2}\) are converted into monoanions \(\mathrm{N}_{2}^{-}\) and \(\mathrm{O}_{2}^{-}\)respectively, which of the following statements is wrong? [AIPMT 1997]
CorrectIncorrectHint
(d) In \(\mathrm{O}_{2}\) bond, the order is 2 and in \(\mathrm{O}_{2}^{-}\)bond, the order is \(1.5\).
-
Question 75 of 134
75. Question
The bond length between hybridised carbon atom and other carbon atom is minimum in [AIPMT 1996]
CorrectIncorrectHint
(b) The \(\mathrm{C}-\mathrm{C}\) bond length \(=1.54 Å, \mathrm{C}=\mathrm{C}\) bond length \(=1.34 Å\) and \(\mathrm{C} \equiv \mathrm{C}\) bond length \(=1.20 Å\). Since propyne has a triple bond, therefore it has minimum bond length.
-
Question 76 of 134
76. Question
Which of the following has \(s p^{2}\)-hybridisation? [AIPMT 1996]
CorrectIncorrectHint
(d) \(\mathrm{BeCl}_{2}\) and \(\mathrm{C}_{2} \mathrm{H}_{2}\) have \(s p\)-hybridisation and \(\mathrm{C}_{2} \mathrm{H}_{6}\) has \(s p^{3}\)-hybridisation.
-
Question 77 of 134
77. Question
Which of the following species is paramagnetic? [AIPMT 1995]
CorrectIncorrectHint
(d) Paramagnetism is caused by the presence of atoms, ions or molecules with unpaired electrons,
\(
\text { i.e }: \dot{\mathrm{N}}=\ddot{\mathrm{O}} \text { : }
\) -
Question 78 of 134
78. Question
The correct order of the \(\mathrm{O}-\mathrm{O}\) bond length in \(\mathrm{O}_{2}, \mathrm{H}_{2} \mathrm{O}_{2}\) and \(\mathrm{O}_{3}\) is [AIPMT 1995]
CorrectIncorrectHint
(b) Bond length of \(\mathrm{O}-\mathrm{O}\) in \(\mathrm{O}_{2}\) is \(1.21 Å\) \((\mathrm{O}=\mathrm{O})\); in \(\mathrm{H}_{2} \mathrm{O}_{2}\) is \(1.48 Å(\mathrm{HO}-\mathrm{HO})\) and in \(\mathrm{O}_{3}\) is
\(
1.28 Å\left(\begin{array}{c}
\mathrm{O}=\mathrm{O} \\
\downarrow \\
\mathrm{O}
\end{array}\right) \text {. }
\) -
Question 79 of 134
79. Question
The ground state electronic configuration of valence shell electrons in nitrogen molecule \(\left(\mathrm{N}_{2}\right)\) is written as \(K K, \sigma 2 s^{2}, \sigma^{*} 2 s^{2}, \pi 2 p_{x}^{2}=\) \(\pi 2 p_{y}{ }^{2} \sigma 2 p_{z}{ }^{2}\). Hence the bond order in nitrogen molecule is [AIPMT 1995]
CorrectIncorrectHint
(b) Number of electrons in bonding orbitals \(N_{b}=10\) and number of electrons in antibonding orbitals \(N_{a}=4\).
Therefore bond order \(=1 / 2\left(N_{b}-N_{a}\right)=1 / 2(10-4)=3\)
-
Question 80 of 134
80. Question
Which of the following molecules has the highest bond order? [AIPMT 1994]
CorrectIncorrectHint
(c) The bond order of \(\mathrm{O}_{2}^{+}=2.5, \mathrm{O}_{2}^{2-}=1\), \(\mathrm{O}_{2}{ }^{-}=1.5\) and that of \(\mathrm{O}_{2}=2\).
-
Question 81 of 134
81. Question
Which of the following molecule does not possess a permanent dipole moment? [AIPMT 1994]
CorrectIncorrectHint
(a) The structure of \(\mathrm{CS}_{2}\) is linear and therefore it does not have permanent dipole moment. It is represented as \(\mathrm{S}=\mathrm{C}=\mathrm{S}\)
-
Question 82 of 134
82. Question
The table shown below gives the bond dissociation energies \(\left(E_{\text {diss }}\right)\) for single covalent bonds of carbon (C) atoms with element \(A\), \(B, C\) and \(D\). Which element has the smallest atoms? [AIPMT 1994]
\(
\begin{array}{|l|c|}
\hline \text { Bond } & E_{\text {diss }}\left(\mathrm{kJ} \mathrm{mol}^{-1}\right) \\
\hline \mathrm{C}-A & 240 \\
\hline \mathrm{C}-B & 328 \\
\hline \mathrm{C}-\mathrm{C} & 276 \\
\hline \mathrm{C}-\mathrm{D} & 485 \\
\hline
\end{array}
\)CorrectIncorrectHint
(b) Smaller the atom, stronger is the bond, and greater the bond dissociation energy. Therefore the bond \(C-D\) has the greatest energy or smallest atoms.
-
Question 83 of 134
83. Question
Among the following which compound will show the highest lattice energy? [AIPMT 1993]
CorrectIncorrectHint
(b) For compounds containing ions of same charge, lattice energy increases as the size of ions decreases. Thus, NaF has highest lattice energy.
-
Question 84 of 134
84. Question
Which one of the following is the correct order of interactions? [AIPMT 1993]
CorrectIncorrectHint
(b) The strength of interaction follow the order van der Waals’ < hydrogen-bond \(<\) dipole-dipole \(<\) covalent. It is so because bond length of H-bond is larger than that of a covalent bond.
And also covalent bond is strongest because, the greater the extent of overlapping, the stronger is the bond formed.
-
Question 85 of 134
85. Question
Which one of the following has the shortest carbon-carbon bond length? [AIPMT 1992]
CorrectIncorrectHint
(c) There is a triple bond in ethyne molecule \((\mathrm{H}-\mathrm{C} \equiv \mathrm{C}-\mathrm{H})\) and due to this triple bond, carboncarbon bond distance is shortest in ethyne.
-
Question 86 of 134
86. Question
Which structure is linear? [AIPMT 1992]
CorrectIncorrectHint
(b) \(\mathrm{CO}_{2}\) molecule is \(s p\)-hybridised and thus it is linear, while \(\mathrm{CO}_{3}{ }^{2-}\) is planar ( \(s p^{2}\)-hybridised), \(\mathrm{SO}_{2}\) is an angular molecule with \(s p^{2}\) hybridisation \(\mathrm{SO}_{4}^{2-}\) is tetrahedral ( \(s p^{3}\)-hybridised).
-
Question 87 of 134
87. Question
Strongest hydrogen bond is shown by [AIPMT 1997]
CorrectIncorrectHint
(c) HF shows strongest H-bonds because fluorine is most electronegative.
-
Question 88 of 134
88. Question
In compound \(X\), all the bond angles are exactly \(109^{\circ} 28^{\prime}, X\) is [AIPMT 1991]
CorrectIncorrectHint
(b) As all \(\mathrm{C}-\mathrm{Cl}\) bonds are directed towards the corner of a regular tetrahedron.
-
Question 89 of 134
89. Question
Among LiCl, \(\mathrm{BeCl}_{2}, \mathrm{BCl}_{3}\) and \(\mathrm{CCl}_{4}\), the covalent bond character follows the order [AIPMT 1990]
CorrectIncorrectHint
(c) Along the period, electronegativity \((E N)\) increases and hence as we move from \(\mathrm{Li} \rightarrow \mathrm{Be} \rightarrow \mathrm{B} \rightarrow \mathrm{C}\), the electronegativity increases.
\(\text { Thus } \mathrm{LiCl}<\mathrm{BeCl}_{2}<\mathrm{BCl}_{3}<\mathrm{CCl}_{4} \text { is correct. }\)
-
Question 90 of 134
90. Question
The complex ion \(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\) is formed by \(s p^{3} d^{2}\) hybridisation. Hence the ion should possess [AIPMT 1990]
CorrectIncorrectHint
(a) According to VSEPR theory, a molecule with 6 bond pairs must be octahedral.
-
Question 91 of 134
91. Question
Which statement is NOT correct? [AIPMT 1990]
CorrectIncorrectHint
(a) A sigma bond is formed by the end-to-end overlapping, so the extent of overlapping is more in case of sigma bond and hence sigma bond is stronger than pi-bond
-
Question 92 of 134
92. Question
which one shows maximum hydrogen bonding? [AIPMT 1990]
CorrectIncorrectHint
(a) \(\mathrm{H}_{2} \mathrm{O}\) shows maximum H-bonding because each \(\mathrm{H}_{2} \mathrm{O}\) molecule is linked to four \(\mathrm{H}_{2} \mathrm{O}\) molecules through H-bonds.
-
Question 93 of 134
93. Question
Linear combination of two hybridized orbitals belonging to two atoms and each having one electron leads to the formation of [AIPMT 1990]
CorrectIncorrectHint
(a) When two hybridised orbitals of two atoms undergoes linear combination, they form sigma bond.
-
Question 94 of 134
94. Question
Which of the following molecule does not have a linear arrangement of atoms? [AIPMT 1990]
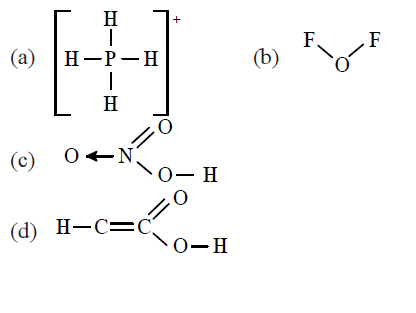 CorrectIncorrect
CorrectIncorrectHint
(d)
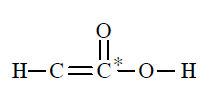
The asterick \((*)\) marked carbon has a valency of 5 and hence this formula is not correct because carbon has a maximum valency of 4 .
-
Question 95 of 134
95. Question
Which of the following molecule does not have a linear arrangement of atoms? [AIPMT 1989]
CorrectIncorrectHint
(a) For linear arrangement of atoms the hybridisation is \(s p\). (bond angle \(=180^{\circ}\) ).
Only \(\mathrm{H}_{2} \mathrm{~S}\) has \(s p^{3}\)-hybridisation and hence it has angular shape while \(\mathrm{C}_{2} \mathrm{H}_{2}, \mathrm{BeH}_{2}\) and \(\mathrm{CO}_{2}\) all involve \(s p\)-hybridisation and hence has linear arrangement of atoms.
-
Question 96 of 134
96. Question
Which of the following does not apply to metallic bond? [AIPMT 1989]
CorrectIncorrectHint
(d) Metallic bonds have electrostatic attraction on all sides and hence do not have directional characteristics.
-
Question 97 of 134
97. Question
In which one of the following molecules the central atom can be said to adopt \(s p^{2}\) hybridization? [AIPMT 1989]
CorrectIncorrectHint
(b) \(\mathrm{BF}_{3} \text { involves } s p^{2} \text {-hybridisation }\)
-
Question 98 of 134
98. Question
\(\mathrm{H}_{2} \mathrm{O}\) has a net dipole moment while \(\mathrm{BeF}_{2}\) has zero dipole moment because [AIPMT 1989]
CorrectIncorrectHint
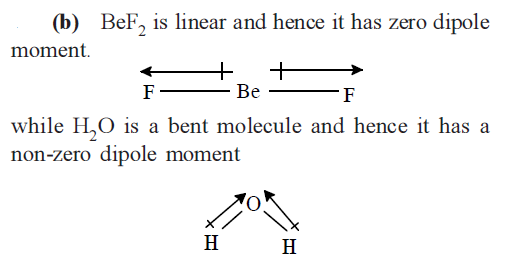
-
Question 99 of 134
99. Question
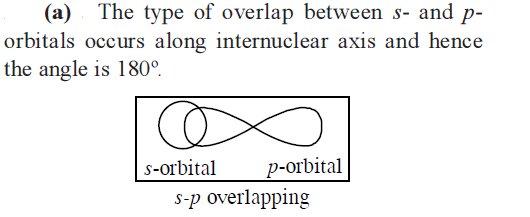
The angle between the overlapping of one \(s\)-orbital and one \(p\)-orbital is [AIPMT 1988]
CorrectIncorrectHint
-
Question 100 of 134
100. Question
Equilateral shape has [AIPMT 1988]
CorrectIncorrectHint
(b) Equilateral or triangular planar shape involves \(s p^{2}\) hybridisation. e.g. \(-\mathrm{BCl}_{3}\).
-
Question 101 of 134
101. Question
The correct sequence of bond enthalpy of ‘ \(\mathrm{C} \longrightarrow \mathrm{X}\) ‘ bond is [NEET 2021]
CorrectIncorrectHint
(b) On moving down the group from \(\mathrm{F}\) to \(\mathrm{I}\), the size of atom increases. Order of the size of halogen atoms is \(\mathrm{I}>\mathrm{Br}>\mathrm{Cl}>\mathrm{F}\).
So, the bond length of \(C-X\) bond also increases from \(\mathrm{F}\) to \(\mathrm{I}\) and hence, the bond enthalpy decreases from \(\mathrm{F}\) to \(\mathrm{I}\).
Correct order of bond length of \(C-X\) bond is
\(
\mathrm{H}_{3} \mathrm{C}-\mathrm{l}>\mathrm{H}_{3} \mathrm{C}-\mathrm{Br}>\mathrm{H}_{3} \mathrm{C}-\mathrm{Cl}>\mathrm{H}_{3} \mathrm{C}-\mathrm{F} \text {. }
\)
Correct order of bond enthalpy is
\(
\mathrm{H}_{3} \mathrm{C}-\mathrm{F}>\mathrm{H}_{3} \mathrm{C}-\mathrm{Cl}>\mathrm{CH}_{3}-\mathrm{Br}>\mathrm{H}_{3} \mathrm{C}-\mathrm{I} \text {. }
\) -
Question 102 of 134
102. Question
Which of the following molecules is non-polar in nature? [NEET 2021]
CorrectIncorrectHint
(c) \(\mathrm{SbCl}_{5}\) Hybridisation \(=\frac{1}{2} \times 10=5\left(s p^{3} d\right)\)
Shape \(=\) Trigonal bipyramidal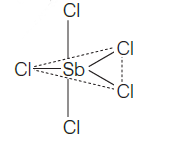
Dipole moment, \(\mu=0\)
\(\mathrm{SbCl}_{5}\) is non-polar in nature. -
Question 103 of 134
103. Question
Match List-I with List-II.
\(
\begin{array}{|l|l|l|l|}
\hline & \text { List – I } & & \text { List – II } \\
\hline \text { (a) } & \mathrm{PCl}_{5} & \text { (i) } & \text { Square pyramidal } \\
\hline \text { (b) } & \mathrm{SF}_{6} & \text { (ii) } & \text { Trigonal planar } \\
\hline \text { (c) } & \mathrm{BrF}_{5} & \text { (iii) } & \text { Octahedral } \\
\hline \text { (d) } & \mathrm{BF}_{3} & \text { (iv) } & \text { Trigonal bipyramidal } \\
\hline
\end{array}
\)Choose the correct answer from the options given below
CorrectIncorrectHint
(c) \(P C l_{5}\) – Trigonal bipyramidal ( 5 bond pairs)
\(S F_{6}\) – Octahedral ( 6 bond pairs)
\(\mathrm{BrF}_{5}\) – Square pyramidal ( 5 bond pairs \(+1\) lone pair)
\(B F_{3}\) – Trigonal planar ( 3 bond pairs) -
Question 104 of 134
104. Question
\(\mathrm{BF}_{3}\) is planar and electron deficient compound. Hybridisation and number of electrons around the central atom, respectively are [NEET 2021]
CorrectIncorrectHint
(c)
Hybridisation of a central atom can be calculate by using the formula:
Hybridisation \(=\frac{1}{2}\) [ number of valence
electrons + Number of side atoms –
Positive charge \(+\) Negative charge \(]\)
Electronic configuration of \(B\)
\(
=1 s^{2}, 2 s^{2}, 2 p^{1}
\)
Number of valence electrons in \(B=3\)
electrons in last shell, \(n=2\)
Number of side atoms in
\(\mathrm{BF}_{3}=3 \mathrm{~F}\)-atoms.
So, hybridisation \(=\frac{1}{2}(3+3)=\frac{1}{2} \times 6=3\).
Hybridisation of \(B\) in \(B F_{3}\) is \(s p^{2}\).
Number of electrons around central
atom, \(\mathrm{B}\) in \(\mathrm{BF}_{3}\) is equal to the number of
electrons in three sigma bonds \((B-F)\) i.e.
\(=3 \mathrm{~B}-\mathrm{F}\) bonds \(\times 2\) electrons in one
\(\sigma\)-bond.
\(=6\) electrons -
Question 105 of 134
105. Question
Which of the following set of molecules will have zero dipole moment? [NEET 2020]
CorrectIncorrectHint
(c) \(\mathrm{BF}_{3}, \mathrm{BeF}_{2}, \mathrm{CO}_{2}\) and 1,4 -dichlorobenzene all are symmetrical molecules.
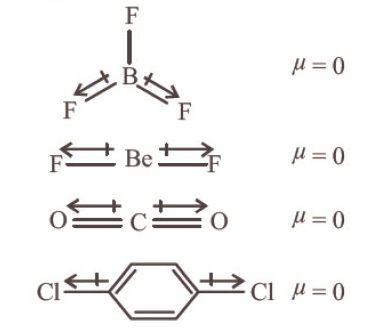
-
Question 106 of 134
106. Question
Which of the following is the correct order of dipole moment? [NEET Odisha 2019]
CorrectIncorrectHint
(c) Dipole moment of a molecule is the vector sum of dipoles of bonds. So based on molecular geometry of following molecules,
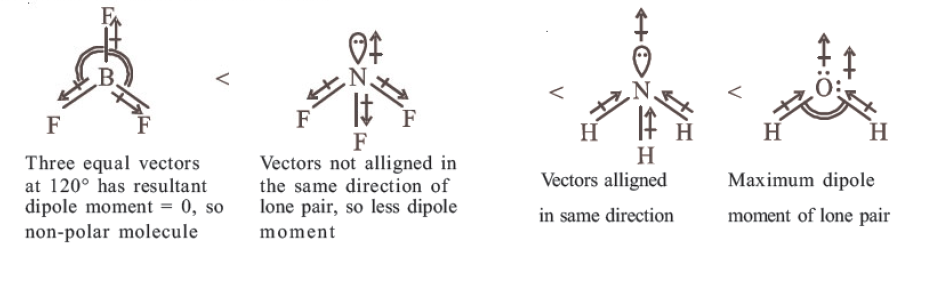
-
Question 107 of 134
107. Question
In the structure of \(\mathrm{ClF}_{3}\), the number of lone pair of electrons on central atom ‘ \(\mathrm{Cl}\) ‘ is [NEET 2018]
CorrectIncorrectHint
(b) The structure of \(\mathrm{ClF}_{3}\) is
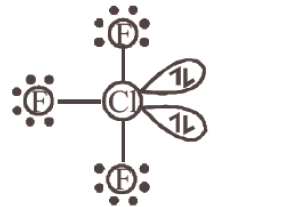
The number of lone pair of electrons on central \(\mathrm{Cl}\) is 2 -
Question 108 of 134
108. Question
Which of the following molecules represents the order of hybridisation \(s p^{2}, s p^{2}, s p, s p\) from left to right atoms? [NEET 2018]
CorrectIncorrectHint
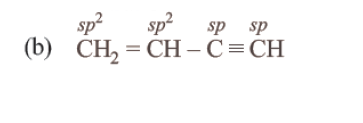
-
Question 109 of 134
109. Question
Identify a molecule which does not exist [NEET 2020]
CorrectIncorrectHint
(d) For \(\mathrm{He}_{2}\) molecule, Electronic configuration is \(\sigma 1 s^{2}, \sigma * 1 s^{2}\)
Bond order \(=\frac{1}{2}\left(N_{b}-N_{a}\right)=\frac{1}{2}(2-2)=0\)
Since, bond order of \(\mathrm{He}_{2}\) is zero, so it does not exist. -
Question 110 of 134
110. Question
Which of the following is paramagnetic? [NEET Odisha 2019]
CorrectIncorrectHint
(a) Molecular orbital configuration of \(\mathrm{O}_{2}\) is given as :
\(
\begin{aligned}
&\mathrm{O}_{2}\left(16 \mathrm{e}^{-}\right): \sigma 1 \mathrm{~s}^{2} \sigma * 1 \mathrm{~s}^{2} \sigma 2 \mathrm{~s}^{2} \sigma * 2 \mathrm{~s}^{2} \sigma 2 p_{z}^{2} \\
&\pi 2 p_{x}^{2}=\pi 2 p_{y}^{2} \pi^{*} 2 p_{x}^{1}=\pi * 2 p_{y}^{1}
\end{aligned}
\)
So, in \(\mathrm{O}_{2}\) molecule, there are two (2) unpaired electrons, so, it is a “paramagnetic” substance in nature. -
Question 111 of 134
111. Question
The manganate and permanganate ions are tetrahedral, due to: [NEET 2019]
CorrectIncorrectHint
(c) Only \(\pi\) bond is present in \(\mathrm{C}_{2}\) molecule.
\(
\sigma 1 s^{2} \sigma^{*} 1 s^{2} \sigma 2 s^{2} \sigma^{*} 2 s^{2} \pi 2 p_{x}^{2}=\pi 2 p_{y}^{2}
\) -
Question 112 of 134
112. Question
Consider the following species:
\(\mathrm{CN}^{+}, \mathrm{CN}^{-}, \mathrm{NO~} {\text {and } \mathrm{CN}}\)
Which one of these will have the highest bond order?[NEET 2018]CorrectIncorrectHint
(b)
\(\mathrm{CN}^{+}\): Valence electrons \(=4+5-1=8\)
\(
\begin{array}{l}
{\left[(\sigma 2 \mathrm{~s})^2\left(\sigma^* 2 \mathrm{~s}\right)^2(\sigma 2 \mathrm{p})^2(\pi 2 \mathrm{p})^2\right.} \\
\mathrm{BO}=\frac{1}{2} \times(4-0)=2
\end{array}
\)\(\mathrm{NO}\): Valence electrons \(=5+6=11\)
\(
\begin{array}{l}
(\sigma 2 \mathrm{~s})^2\left(\sigma^* 2 \mathrm{~s}\right)^2(\sigma 2 \mathrm{p})^2(\pi 2 \mathrm{p})^4\left(\pi^* 2 \mathrm{p}\right)^1 \\
\mathrm{BO}=\frac{1}{2} \times(6-1)=2.5
\end{array}
\)
\(\mathrm{CN}\) : Valence electrons \(=4+5=9\)
\(
\begin{array}{l}
(\sigma 2 \mathrm{~s})^2\left(\sigma^* 2 \mathrm{~s}\right)^2(\sigma 2 \mathrm{p})^2(\pi 2 \mathrm{p})^3 \\
\mathrm{BO}=\frac{1}{2} \times(5-0)=2.5
\end{array}
\)
\(\mathrm{CN}^{-}\): Valence electrons \(=4+5+1=10\)
\(
(\sigma 2 \mathrm{~s})^2\left(\sigma^* 2 \mathrm{~s}\right)^2(\sigma 2 \mathrm{p})^2(\pi 2 \mathrm{p})^4
\)
\(
\mathrm{BO}=\frac{1}{2} \times(6-\mathrm{o})=3
\)Hence, highest bond order is for \(\mathrm{CN}^{-}\)
-
Question 113 of 134
113. Question
The correct order of dipole moments for molecules \(\mathrm{NH}_3, \mathrm{H}_2 \mathrm{S}, \mathrm{CH}_4\) and \(\mathrm{HF}\), is: [NEET 2023 Manipur]
CorrectIncorrectHint
(d)
\(
\mathrm{HF}>\mathrm{NH}_3>\mathrm{H}_2 \mathrm{S}>\mathrm{CH}_4 \text { (Non-polar) }
\) -
Question 114 of 134
114. Question
Which one of the following represents all isoelectronic species? [NEET 2023 Manipur]
CorrectIncorrectHint
(d) For Isoelectronic species total numbers electrons are same \(\mathrm{Ca}^{+2}, \mathrm{Ar}, \mathrm{K}^{+}, \mathrm{Cl}^{-} \rightarrow 20\) electrons
-
Question 115 of 134
115. Question
Which one of the following statements is incorrect related to Molecular Orbital Theory? [NEET 2023 Manipur]
CorrectIncorrectHint
(c) In the formation of BMO, the two electron waves of the bonding atoms reinforce each other due to constructive interference. Molecular orbitals obtained from \(2 \mathrm{P}_{\mathrm{x}}\) and \(2 \mathrm{P}_{\mathrm{y}}\) orbitals are ‘unsymmetrical’ around bond axis.
-
Question 116 of 134
116. Question
Given below are two statements: [NEET 2023]
Statement I: Hydrated chlorides and bromides of \(\mathrm{Ca}, \mathrm{Sr}\) and \(\mathrm{Ba}\) on heating undergo hydrolysis.
Statement II : Hydrated chlorides and bromides of Be and \(\mathrm{Mg}\) on heating undergo dehydration.
In the light of the above statements, choose the correct answer from the options given below :CorrectIncorrectHint
(d) Hydrated chlorides and Bromides of \(\mathrm{Ca}, \mathrm{Sr}\) and \(\mathrm{Ba}\) are lonic so undergo dehydration after heating. Hydrated chlorides and Bromides of \(\mathrm{Be}\) and \(\mathrm{Mg}\) are covalent so undergo hydrolysis on Heating.
-
Question 117 of 134
117. Question
Amongst the following, the total number of species NOT having eight electrons around central atom in its outer most shell, is \(
\mathrm{NH}_3, \mathrm{AlCl}_3, \mathrm{BeCl}_2, \mathrm{CCl}_4, \mathrm{PCl}_5 \text { : }\) [NEET 2023]CorrectIncorrectHint
(d) Total number of species =3
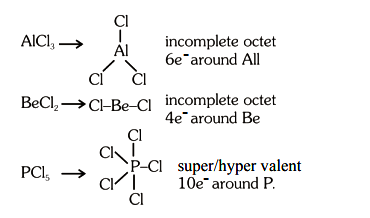
-
Question 118 of 134
118. Question
The correct order of energies of molecular orbitals of \(\mathrm{N}_2\) molecule, is [NEET 2023]
CorrectIncorrectHint
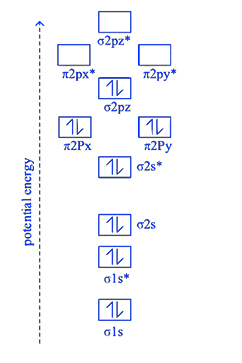
(d) Molecular orbital (energy) diagram / sequence of \(\mathrm{N}_2\)
-
Question 119 of 134
119. Question
Taking stability as the factor, which one of the following represents correct relationship? [NEET 2023]
CorrectIncorrectHint
(c) The stability of a compound is often related to the charge on the central atom and the nature of its bonding with the ligands. In this case, \(\mathrm{TlI}\left(\mathrm{Tl}^{+1}\right)\) refers to thallium(I) ions, and \(\mathrm{TlI}_3\left(\mathrm{Tl}^{3+}\right)\) refers to thallium(III) ions.
Generally, lower oxidation states (e.g., \(\mathrm{Tl}^{+}\)) are more stable than higher oxidation states (e.g., \(\mathrm{Tl}^{3+}\) ) for heavy elements like thallium. This is due to the presence of additional electron shells that can provide increased shielding and stability for lower oxidation states.
Option [C] \(\mathrm{TlI}>\mathrm{TlI}_3\) reflects this principle and correctly represents the relationship based on stability.
Note: due to inert pair effect \(\mathrm{T} l^{+}\)is more stable than \(\mathrm{T} l^{+3}\).
-
Question 120 of 134
120. Question
Intermolecular forces are forces of attraction and repulsion between interacting particles that will include:
A. dipole – dipole forces.
B. dipole – induced dipole forces
C. hydrogen bonding
D. covalent bonding
E. dispersion forces
Choose the most appropriate answer from the options given below : [NEET 2023]CorrectIncorrectHint
(b) Intermolecular forces means force of attraction between two or more molecules
dipole-dipole (attraction between two or more polar molecules).
Dipole induced dipole (attraction between polar and non-polar molecules)
Hydrogen bonding (it is a special type of dipole-dipole and ion-dipole attraction)
Dispersion forces (mainly acts between non polar molecules).
Covalent bonding (acts between atom not between molecules) -
Question 121 of 134
121. Question
Match List-I with List-II :
\(
\begin{array}{|l|l|}
\hline \begin{array}{l}
\text { List-I } \\
\text { (Molecules) }
\end{array} & \begin{array}{l}
\text { List-II } \\
\text { (Shape) }
\end{array} \\
\hline \text { (a) } \mathrm{NH}_3 & \text { (i) Square pyramidal } \\
\hline \text { (b) } \mathrm{ClF}_3 & \text { (ii) Trigonal bipyramidal } \\
\hline \text { (c) } \mathrm{PCl}_5 & \text { (iii) Trigonal pyramidal } \\
\hline \text { (d) } \mathrm{BrF}_5 & \text { (iv) T-shape } \\
\hline
\end{array}
\)
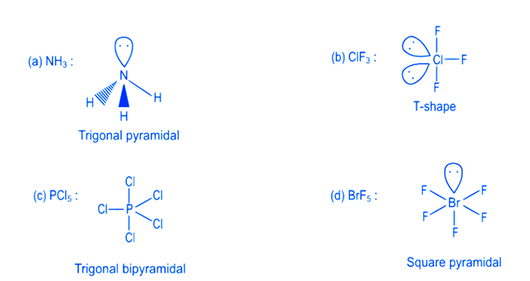
Choose the correct answer from the options given below: [NEET 2022 Phase 2]CorrectIncorrectHint
(c)
-
Question 122 of 134
122. Question
The correct order of bond angles in the following compounds/species is : [NEET 2022 Phase 2]
CorrectIncorrectHint
(a)
\(\mathrm{CO}_2 \Rightarrow \mathrm{sp}^2\) hybridisation, bond angle \(=180^{\circ}\)
\(\mathrm{NH}_4^{+} \Rightarrow \mathrm{sp}^3\) hybridisation, bond angle \(=109^{\circ} 28^{\prime}\)
\(\mathrm{NH}_3 \Rightarrow \mathrm{sp}^3\) hybridisation with one lone pair on central atom, bond angle \(\simeq 107^{\circ}\)
\(\mathrm{H}_2 \mathrm{O} \Rightarrow \mathrm{sp}^3\) hybridisation with two lone pairs on central atom, bond angle \(\simeq 104.5^{\circ}\) -
Question 123 of 134
123. Question
Given below are two statements; one is labelled as Assertion A and the other is labelled as Reason(R).
Assertion \((\mathrm{A})\) : \(\mathrm{ICl}\) is more reactive than \(\mathrm{I}_2\).
Reason(R): I-CI bond is weaker than I-I bond.
In the light of the above statements, choose the most appropriate answer from the options given below :[NEET 2022 Phase 1]CorrectIncorrectHint
(a) In general, interhalogen compounds are more reactive than halogens (except fluorine). This is because \(X-X^{\prime}\) bond in interhalogens is weaker than \(\mathrm{X}-\mathrm{X}\) bond in halogens excepts \(\mathrm{F}-\mathrm{F}\) bond. Therefore \(\mathrm{I}-\mathrm{Cl}\) is more reactive than \(\mathrm{I}_2\) because of weaker \(\mathrm{I}-\mathrm{Cl}\) bond then \(\mathrm{I}\) – \(\mathrm{I}\) bond.
-
Question 124 of 134
124. Question
Amongst the following which one will have maximum ‘lone pair – lone pair’ electron repulsions? [NEET 2022 Phase 1]
CorrectIncorrectHint
(d)
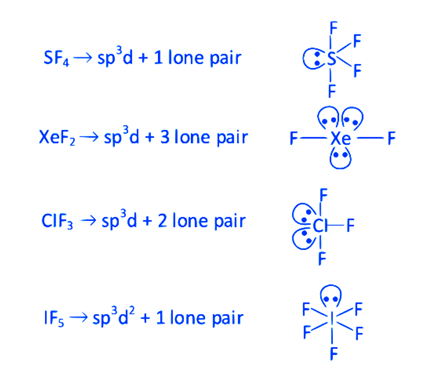
\(\mathrm{XeF}_2\) having maximum lone pairs, so, it has maximum ‘Ione pair-lone pair’ electron repulsions.
-
Question 125 of 134
125. Question
Which amongst the following is incorrect statement? [NEET 2022 Phase 1]
CorrectIncorrectHint
(d)
\(\mathrm{O}_2^{+}\)ion is having 15 electrons, so it contain one unpaired electron. Hence it is paramagnetic in nature.
\(
\sigma 1 s^2 \sigma^* 1 s^2 \sigma 2 s^2 \sigma^* 2 s^2 \sigma 2 p_z^2
\)
\(
\begin{array}{cc}
\pi 2 p_x^2 & \pi^* 2 p_x^1 \\
\mid & \mid \\
\pi 2 p_y^2 & \pi 2 p_y
\end{array}
\)
Due to one unpaired electron in \(\pi * 2 \mathrm{p}\) molecular orbital, \(\mathrm{O}_2^{+}\)is a paramagnetic ion. -
Question 126 of 134
126. Question
Which of the following molecules has “NON ZERO” dipole moment value? [NEET 2024 (Re-Examination)]
CorrectIncorrectHint
(b)
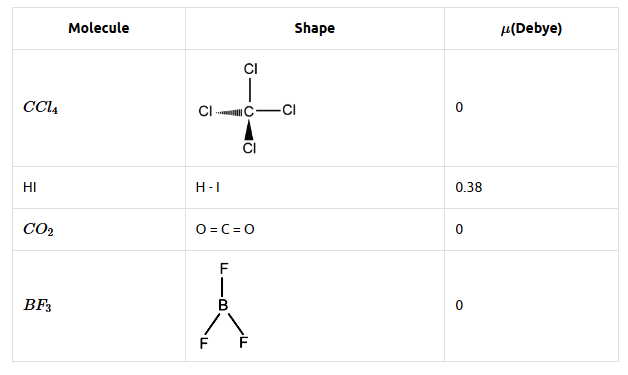
Note: Dipole moment of a molecule depends both on shape and bond dipole.
-
Question 127 of 134
127. Question
Arrange the following compounds in increasing order of their solubilities in chloroform: \(NaCl , CH _3 OH\), cyclohexane, \(CH _3 CN\) [NEET 2024 (Re-Examination)]
CorrectIncorrectHint
(a) Since \(CHCl _3\) is an organic solvent so, covalent (non-polar) compounds will be more soluble in it. As the dipole moment of solute increases, solubility in chloroform decreases. Hence increasing order of solubility.
\(
NaCl < CH _3 CN < CH _3 OH <\text { Cyclohexane }
\) -
Question 128 of 134
128. Question
Identify the incorrect statement about \(PCl _5\). [NEET 2024 (Re-Examination)]
CorrectIncorrectHint
(b) It is \(s p^3 d\) hybridised with axial to equatorial angle of \(90^{\circ}\) and equatorial bond angles of \(120^{\circ}\). It has five \(P – Cl\) sigma bonds. Axial bonds are longer than equatorial bonds.
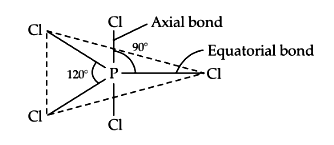
-
Question 129 of 134
129. Question
Match List-I with List-II: [NEET 2024 (Re-Examination)]
\(
\begin{array}{|l|l|l|l|}
\hline & \text { List-I (Bond) } & & \text { List-II (Bond Enthalpy) } \left( kJ mol ^{-1}\right) \\
\hline \text { (A) } & HCl & \text { (I) } & 435.8 \\
\hline \text { (B) } & N _2 & \text { (II) } & 498 \\
\hline \text { (C) } & H _2 & \text { (III) } & 946.0 \\
\hline \text { (D) } & O _2 & \text { (IV) } & 431.0 \\
\hline
\end{array}
\)
Choose the correct answer from the options given below:CorrectIncorrectHint
(d)
\(
\begin{aligned}
&\begin{array}{|c|c|}
\hline \text { Molecule } & \text { Bond enthalpy ( } kJ mol ^{-1} \text { ) } \\
\hline HCl & 431.0 \\
\hline N _2 & 946.0 \\
\hline H _2 & 435.8 \\
\hline O _2 & 498 \\
\hline
\end{array}\\
&\text { Molecule }\\
&\text { Bond enthalpy ( } kJ mol ^{-1} \text { ) }\\
&HCl\\
&431.0\\
&N _2\\
&946.0\\
&H _2\\
&435.8\\
&O _2\\
&498
\end{aligned}
\) -
Question 130 of 134
130. Question
Match List-I with List-II: [NEET 2024]
\(
\begin{array}{|l|l|l|l|}
\hline & \text { List-I (Molecule) } & & \text { List-II (Number and types of bond/s between two carbon atoms) } \\
\hline \text { A. } & \text { ethane } & \text { I. } & \text { one } \sigma \text {-bond and two } \pi \text {-bonds } \\
\hline \text { B. } & \text { ethene } & \text { II. } & \text { two } \pi \text {-bonds } \\
\hline \text { C. } & \text { carbon molecule, } C _2 & \text { III. } & \text { one } \sigma \text {-bond } \\
\hline \text { D. } & \text { ethyne } & \text { IV. } & \text { one } \sigma \text {-bond and one } \pi \text {-bond } \\
\hline
\end{array}
\)
Choose the correct answer from the options given below:CorrectIncorrectHint
(c)
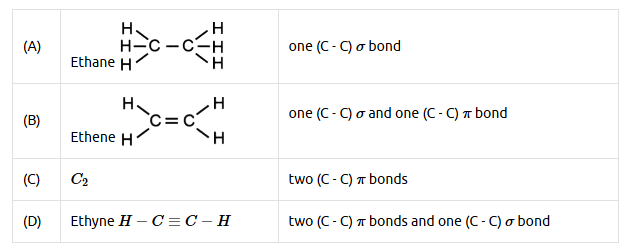
-
Question 131 of 134
131. Question
Given below are two statements:
Statement I: The boiling point of hydrides of Group 16 elements follow the order \(H _2 O > H _2 Te > H _2 Se > H _2 S\)
Statement II: On the basis of molecular mass, \(H _2 O\) is expected to have lower boiling point than the other members of the group but due to the presence of extensive H -bonding in \(H _2 O\), it has higher boiling point.
In the light of the above statements, choose the correct answer from the options given below: [NEET 2024]CorrectIncorrectHint
(a) The given question involves understanding the boiling points of Group 16 hydrides and the effect of molecular mass and hydrogen bonding on these boiling points.
Statement I claims that the boiling points of Group 16 hydrides follow this order:
\(
H _2 O > H _2 Te > H _2 Se > H _2 S
\)This statement is indeed true. Water \(\left( H _2 O \right)\) has a much higher boiling point compared to the other hydrides in Group 16. This anomaly primarily arises due to the extensive hydrogen bonding present in water, which greatly increases its boiling point. In the absence of such strong hydrogen bonding, heavier molecules like \(H _2 Te , H _2 Se\), and \(H _2 S\) would typically have higher boiling points due to increased van der Waals forces (attributable to larger molecular mass and size). But in this case, \(H _2 O\) surpasses them because hydrogen bonds are much stronger than van der Waals forces.
Statement II states that based on molecular mass alone, \(H _2 O\) should have a lower boiling point than the other Group 16 hydrides but has a higher boiling point due to extensive hydrogen bonding. This statement is also true. Water, having a lower molecular mass, would typically have a lower boiling point if we consider only molecular mass. However, the strong hydrogen bonds between water molecules contribute to a much higher boiling point. Hydrogen bonding significantly impacts physical properties like boiling point because it requires more energy to break these bonds during the phase change from liquid to gas.
In summary, based on the analysis of the two statements:
Option A: Both Statement I and Statement II are true is the correct answer. Each statement is correct, and both relate accurately to the anomalous behavior of water among the hydrides of Group 16 elements. -
Question 132 of 134
132. Question
Intramolecular hydrogen bonding is present in [NEET 2024]
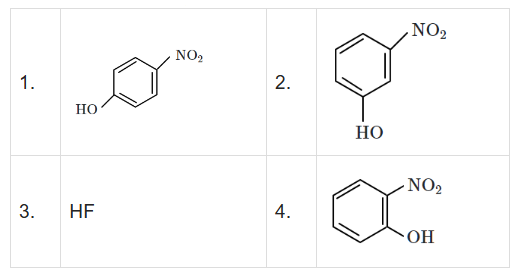 CorrectIncorrect
CorrectIncorrectHint
(d) In o-nitrophenol intramolecular H-bonding is present.
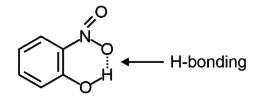
-
Question 133 of 134
133. Question
Match List-I with List-II: [NEET 2024]
\(
\begin{array}{|l|l|l|l|}
\hline & \text { List-I (Compound) } & & \text { List-II ((Shape/geometry)) } \\
\hline \text { A. } & NH _3 & \text { I. } & \text { Trigonal Pyramidal } \\
\hline \text { B. } & BrF _5 & \text { II. } & \text { Square Planar } \\
\hline \text { C. } & XeF _4 & \text { III. } & \text { Octahedral } \\
\hline \text { D. } & SF _6 & \text { IV. } & \text { Square Pyramidal } \\
\hline
\end{array}
\)
Choose the correct answer from the options given below:CorrectIncorrectHint
(a)
\(NH _3 \Rightarrow s p^3\) hybridised with 1 lone pair.
Structure will be Trigonal Pyramidal.
\(BrF _5 \Rightarrow s p^3 d^2\) hybridised with 1 lone pair.
Structure will be Square Pyramidal.
\(XeF _4 \Rightarrow s p^3 d^2\) with two lone pairs.
Structure will be Square Planar.
\(SF _6 \Rightarrow s p^3 d^2\) with no lone pair.
Structure will be Octahedral.
A-I, B-IV, C-II, D-III -
Question 134 of 134
134. Question
Identify the correct answer. [NEET 2024]
CorrectIncorrectHint
(d)
(1) In ozone; there are two resonating structures.