4.2 Ionic or Electrovalent Bond
Ionic or Electrovalent Bond
The bond formed as a result of electrostatic attraction between positive and negative ions is called ionic bond. Ionic bonds are formed readily between elements with low IE and elements with high -ve value of electron gain enthalpy.
Factors favouring the formation of ionic bond:
- Ionisation energy (IE)
- Electron affinity
- Lattice energy
Note: A qualitative measure of the stability of an ionic compound is provided by its lattice enthalpy which is defined as the energy required to completely separate one mole of a solid ionic compound into gaseous constituent ions.
From the Kossel and Lewis treatment of the formation of an ionic bond, it follows that the formation of ionic compounds would primarily depend upon:
- The ease of formation of the positive and negative ions from the respective neutral atoms;
- The arrangement of the positive and negative ions in the solid, that is, the lattice of the crystalline compound.
The formation of a positive ion involves ionization, i.e., the removal of electron(s) from the neutral atom and that of the negative ion involves the addition of electron(s) to the neutral atom.
\(
\mathrm{M}(\mathrm{g}) \rightarrow \mathrm{M}^{+}(\mathrm{g})+\mathrm{e}^{-}; \text { Ionization enthalpy }
\)
\(
\mathrm{X}(\mathrm{g})+\mathrm{e}^{-} \rightarrow \mathrm{X}^{-}(\mathrm{g}); \text { Electron gain enthalpy }
\)
\(
\mathrm{M}^{+}(\mathrm{g})+\mathrm{X}^{-}(\mathrm{g}) \rightarrow \mathrm{MX}(\mathrm{s})
\)
- The electron gain enthalpy, \(\Delta_{e g} H\), is the enthalpy change when a gas phase atom in its ground state gains an electron. The electron gain process may be exothermic or endothermic. The ionization, on the other hand, is always endothermic. Electron affinity is the negative of the energy change accompanying electron gain.
- Obviously, ionic bonds will be formed more easily between elements with comparatively low ionization enthalpies and elements with comparatively high negative value of electron gain enthalpy.
- Most ionic compounds have cations derived from metallic elements and anions from non-metallic elements. The ammonium ion, \(\mathrm{NH}_4^{+}\)(made up of two nonmetallic elements) is an exception. It forms the cation of a number of ionic compounds.
Ionic compounds in the crystalline state (Rock Salt Structure)
Ionic compounds in the crystalline state consist of orderly three-dimensional arrangements of cations and anions held together by coulombic interaction energies. These compounds crystallise in different crystal structures determined by the size of the ions, their packing arrangements and other factors. The crystal structure of sodium chloride, \(\mathrm{NaCl}\) (rock salt), for example, is shown below.
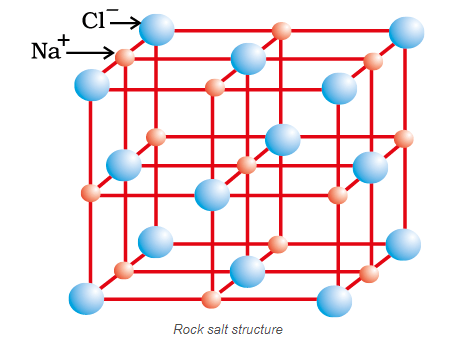
In ionic solids, the sum of the electron gain enthalpy and the ionization enthalpy may be positive but still the crystal structure gets stabilized due to the energy released in the formation of the crystal lattice. For example the ionization enthalpy for \(\mathrm{Na}^{+}(\mathrm{g})\) formation from \(\mathrm{Na}(\mathrm{g})\) is \(495.8 \mathrm{~kJ} \mathrm{~mol}^{-1}\); while the electron gain enthalpy for the change \(\mathrm{Cl}(\mathrm{g})+\mathrm{e}^{-} \rightarrow\) \(\mathrm{Cl}^{-}(\mathrm{g})\) is, \(-348.7 \mathrm{~kJ} \mathrm{~mol}^{-1}\) only. The sum of the two, \(147.1 \mathrm{~kJ} \mathrm{~mol}^{-1}\) is more than compensated for by the enthalpy of lattice formation of \(\mathrm{NaCl}(\mathrm{s})\left(-788 \mathrm{~kJ} \mathrm{~mol}^{-1}\right)\). Therefore, the energy released in the processes is more than the energy absorbed.
Thus a qualitative measure of the stability of an ionic compound is provided by its enthalpy of lattice formation and not simply by achieving octet of electrons around the ionic species in the gaseous state.
Since lattice enthalpy plays a key role in the formation of ionic compounds, let’s study it in the next section.
Lattice Enthalpy
The Lattice Enthalpy of an ionic solid is defined as the energy required to completely separate one mole of a solid ionic compound into gaseous constituent ions.
For example, the lattice enthalpy of \(\mathrm{NaCl}\) is \(788 \mathrm{~kJ} \mathrm{~mol}{ }^{-1}\). This means that \(788 \mathrm{~kJ}\) of energy is required to separate one mole of solid \(\mathrm{NaCl}\) into one mole of \(\mathrm{Na}^{+}(\mathrm{g})\) and one mole of \(\mathrm{Cl}^{-}(\mathrm{g})\) to an infinite distance.
This process involves both the attractive forces between ions of opposite charges and the repulsive forces between ions of like charge. The solid crystal being three-dimensional; it is not possible to calculate lattice enthalpy directly from the interaction of forces of attraction and repulsion only. Factors associated with the crystal geometry have to be included.