3.5 Electronic Configurations of Elements and the Periodic Table
- We have learnt that an electron in an atom is characterised by a set of four quantum numbers, and the principal quantum number \((n)\) defines the main energy level known as shell.
- We have also studied about the filling of electrons into different subshells, also referred to as orbitals (s, \(p, d\), \(f\) ) in an atom.
- The distribution of electrons into orbitals of an atom is called its electronic configuration. An element’s location in the Periodic Table reflects the quantum numbers of the last orbital filled.
(a) Electronic Configurations in Periods
- The value of \(n\), the principal quantum number, for the valence shell is the period of the element.
- Different energy levels can accommodate different numbers of electrons. In other words, successive period in the Periodic Table is associated with the filling of the next higher principal energy level ( \(n=1, n=2\), etc.). It can be readily seen that the number of elements in each period is twice the number of atomic orbitals available in the energy level that is being filled.
- The maximum number of electrons that a given energy level can accommodate is given by \(2 n^2\), where \(n\) is the energy level. So the first energy level (K shell) can hold up to 2 electrons, the second level ( \(\mathrm{L}\) shell) can hold up to 8 electrons, the third level ( \(\mathrm{M}\) shell) can also hold 18 electrons and the fourth level ( \(\mathrm{N}\) shell) can also hold 32 electrons and so on.
- Thus, the first period with \(n=1\) can hold up to 2 elements by filling the lowest level 1s. The elements are Hydrogen and Helium with electronic configurations \(1 s^1\) and \(1 s^2\) This marks the complete filling of \(\mathrm{K}\) shell.
- The second period starts with Lithium and Beryllium which have 3 and 4 electrons and hence the last electrons enter the level \(2 \mathrm{~s}\) and they have an electronic configuration of \(1 s^2 2 s^1\) and \(1 s^2 2 s^2\) This is followed by the start of the \(2 p\) orbital filling. It starts with Boron \(\left(1 s^2 2 s^2 2 p^1\right)\) and ends with Neon \(\left(1 s^2 2 s^2 2 p^6\right)\) which marks the completion of \(L\) shell. Thus, 8 elements are present in the second period.
- The third period ( \(n=3\) ) begins at sodium, and the added electron enters a \(3 s\) orbital. Successive filling of \(3 s\) and \(3 p\) orbitals gives rise to the third period of 8 elements from sodium to argon.
- The fourth period \((n=4)\) starts at potassium, and the added electrons fill up the \(4 s\) orbital. However, we know that \(3 \mathrm{~d}\) orbital is to be filled (known as \(3 d\) transition series of elements.) before filling of \(4 \mathrm{p}\) orbital starts. This starts from scandium \((Z=21)\) which has the electronic configuration \(3 d^1 4 s^2\). The \(3 d\) orbitals are filled at zinc \((Z=30)\) with electronic configuration \(3 d^{10} 4 s^2\). The fourth period ends at krypton with the filling up of the \(4 p\) orbitals. Altogether we have 18 elements in this fourth period.
- The fifth period \((n=5)\) beginning with rubidium is similar to the fourth period and contains the \(4 d\) transition series starting at yttrium \((Z=39)\). This period ends at xenon with the filling up of the \(5 p\) orbitals.
- The sixth period \((n=6)\) contains 32 elements and successive electrons enter \(6 s, 4 f\), \(5 d\) and \(6 p\) orbitals, in the order – filling up of the \(4 f\) orbitals begins with cerium \((Z=58)\) and ends at lutetium \((Z=71)\) to give the \(4 f\)-inner transition series which is called the lanthanoid series.
- The seventh period ( \(n=7\) ) is similar to the sixth period with the successive filling up of the \(7 \mathrm{~s}\), \(5 f, 6 d\) and \(7 p\) orbitals and includes most of the man-made radioactive elements. This period will end at the element with atomic number 118 which would belong to the noble gas family. Filling up of the \(5 f\) orbitals after actinium \((Z=89)\) gives the \(5 f\)–inner transition series known as the actinoid series. The \(4 f-\) and \(5 f\)–inner transition series of elements are placed separately in the Periodic Table to maintain its structure and to preserve the principle of classification by keeping elements with similar properties in a single column.
Example 3.2: How would you justify the presence of 18 elements in the \(5^{\text {th }}\) period of the Periodic Table?
Answer: When \(n=5, l=0,1,2,3\). The order in which the energy of the available orbitals \(4 d, 5 s\) and \(5 p\) increases is \(5 s<4 d<5 p\). The total number of orbitals available are 9. The maximum number of electrons that can be accommodated is 18; and therefore 18 elements are there in the \(5^{\text {th }}\) period.
(b) Groupwise Electronic Configurations
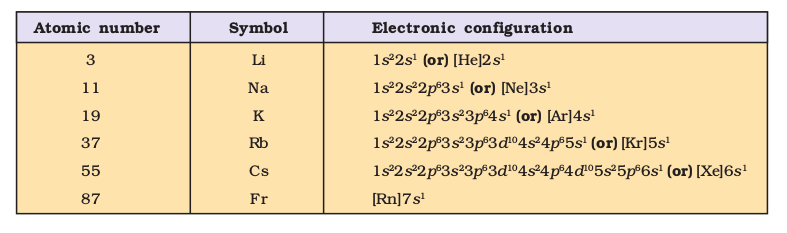
- Elements in the same vertical column or group have similar valence shell electronic configurations, the same number of electrons in the outer orbitals, and similar properties. For example, the Group 1 elements (alkali metals) all have \(n s^1\) valence shell electronic configuration as shown below.
- Thus it can be seen that the properties of an element have periodic dependence upon its atomic number and not on relative atomic mass.