1.8 Mole Concept and Molar Masses
In SI system, mole (symbol, mol) was introduced as the seventh base quantity for the amount of a substance. The mole, symbol mol, is the SI unit of the amount of substance.
One mole contains exactly \(6.02214076 \times 10^{23}\) elementary entities. This number is the fixed numerical value of the Avogadro constant, \(N_{A}\), when expressed in the unit \(\mathrm{mol}^{-1}\) and is called the Avogadro number. The amount of substance, symbol \(n\), of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, or any other particle or specified group of particles.
It may be emphasised that the mole of a substance always contains the same number of entities, no matter what the substance may be. In order to determine this number precisely, the mass of a carbon-12 atom was determined by a mass spectrometer and found to be equal to \(1.992648 \times 10^{-23} \mathrm{~g}\). Knowing that one mole of carbon weighs 12 g, the number of atoms in it is equal to:
\(
\begin{aligned}
&\frac{12 \mathrm{~g} / \mathrm{mol}^{~12} \mathrm{C}}{1.992648 \times 10^{-23} \mathrm{~g} /{ }^{12} \text { C atom }} \\
&=6.0221367 \times 10^{23} \text { atoms } / \mathrm{mol}
\end{aligned}
\)
This number of entities in \(1 \mathrm{~mol}\) is so important that it is given a separate name and symbol. It is known as ‘Avogadro constant‘, or Avogadro number denoted by \(\mathrm{N}_{\mathrm{A}}\) in honour of Amedeo Avogadro. To appreciate the largeness of this number, let us write it with all zeroes without using any powers of ten.
\(
602213670000000000000000
\)
Hence, so many entities (atoms, molecules, or any other particle) constitute one mole of a particular substance.
We can, therefore, say that \(1 \mathrm{~mol}\) of hydrogen atoms \(=6.022 \times 10^{23}\) atoms
\(1 \mathrm{~mol}\) of water molecules \(=6.022 \times 10^{23}\) water molecules
\(1 \mathrm{~mol}\) of sodium chloride \(=6.022 \times 10^{23}\) formula units of sodium chloride
Having defined the mole, it is easier to know the mass of one mole of a substance or the constituent entities. The mass of one mole of a substance in grams is called its molar mass. The molar mass in grams is numerically equal to atomic/molecular/ formula mass in u.
Molar mass of water \(=18.02 \mathrm{~g} \mathrm{~mol}^{-1}\)
Molar mass of sodium chloride \(=58.5 \mathrm{~g} \mathrm{~mol}^{-1}\)
Mole Concept
\(
\text { Mole : Mole is a unit which represents } 6.023 \times 10^{23} \text { particles of same nature. }
\)
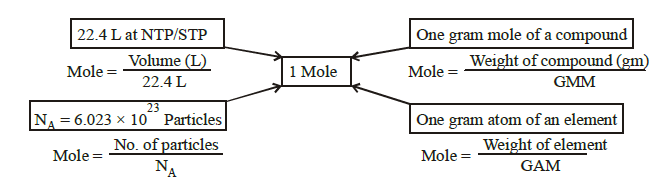
- 1 Mole \(=6.023 \times 10^{23}\) particles.
- 1 Mole of atoms \(=6.023 \times 10^{23}\) Atoms.
- 1 Mole of molecules \(=6.023 \times 10^{23}\) molecules
- 1 Mole of electrons \(=6.023 \times 10^{23}\) electrons.
- The number \(6.023 \times 10^{23}\) is called Avogadro number \(\left(N_A\right)\)
EQUIVALENT WEIGHT
The equivalent weight of a substance (element or compound) is defined as “The number of parts by weight of it, that will combine with or displace directly or indirectly 1.008 parts by weight of hydrogen, 8 parts by weight of oxygen, 35.5 parts by weight of chlorine or the equivalent parts by weight of another element”.
\(
\begin{aligned}
& \text { Eq. wt of elements }=\frac{\text { Molecular mass }}{\text { Acidity of base }} \\
& \text { Eq. wt of elements }=\frac{\text { Molecular mass }}{\text { Basicity of acid }} \\
& \text { Eq. wt of an acid }=\frac{\text { Molecular mass }}{\text { Basicity of acid }} \\
& \text { Eq. wt of a base }=\frac{\text { Molecular mass }}{\text { Acidity of base }} \\
& \text { Equivalent mass for salts } \\
& \qquad=\frac{\text { Formula mass }}{\text { (Valency of cation) (No. of cations) }}
\end{aligned}
\)
Equivalent mass for salts
\(
=\frac{\text { Formula mass }}{\text { (Valency of cation) (No. of cations) }}
\)
Equivalent mass for oxidising agents
\(
=\frac{\text { Formula mass }}{\text { No. of electrons gained per molecule }}
\)
Equivalent mass for reducing agents
\(
=\frac{\text { Formula mass }}{\text { No. of electrons lost per molecule }}
\)