Redox Reactions Quiz-1
Multiple Choice Questions with one Correct Answer
Quiz Summary
0 of 50 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 50 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 50
1. Question
In which of the following pairs, there is greatest difference in the oxidation number of the underlined elements?
CorrectIncorrectHint
(d) O.N. of \(\mathrm{N}\) in \(\mathrm{NO}_2\) and \(\mathrm{N}_2 \mathrm{O}_4\) is +4 difference is zero.
O.N. of \(\mathrm{P}\) in \(\mathrm{P}_2 \mathrm{O}_5\) and \(\mathrm{P}_4 \mathrm{O}_{10}\) is +5 difference is zero
\(
\mathrm{O} . \mathrm{N} \text {. of } \mathrm{N} \text { in } \mathrm{N}_2 \mathrm{O} \text { is }+1 \text { and in } \mathrm{NO} \text { is }+2 \text {. Thedifference is } 1
\)
\(
\text { O.N. of S in } \mathrm{SO}_2 \text { is }+4 \text { and in } \mathrm{SO}_3 \text { is }+6 \text {. The difference is }+2 \text {. }
\) -
Question 2 of 50
2. Question
Which of the following is a redox reaction?
CorrectIncorrectHint
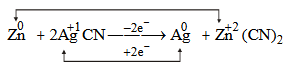
The oxidation state shows a change only in (d)
-
Question 3 of 50
3. Question
Several blocks of magnesium are fixed to the bottom of a ship to
CorrectIncorrectHint
(b) Magnesium provides cathodic protection and prevent rusting or corrosion.
-
Question 4 of 50
4. Question
When \(\mathrm{KMnO}_4\) reacts with acidified \(\mathrm{FeSO}_4\)
CorrectIncorrectHint
\(
{2 \mathrm{~K}} \stackrel{+7}{\mathrm{M}} \mathrm{O}_4+3 \mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{K}_2 \mathrm{SO}_4+2 \stackrel{+2}{\mathrm{Mn} \mathrm{SO}_4}+3 \mathrm{H}_2 \mathrm{O}+5 \mathrm{O}
\)
\(
\stackrel{+2}{2 \mathrm{FeSO}_4}+\mathrm{H}_2 \mathrm{SO}_4+\mathrm{O} \rightarrow \stackrel{+3}{\mathrm{Fe}_2}\left(\mathrm{SO}_4\right)_3+\mathrm{H}_2 \mathrm{O}
\)
O.N. of Mn changes from +7 to +2 (Reduction)
O.N. of Fe changes from +2 to +3 (Oxidation) -
Question 5 of 50
5. Question
Which of the following chemical reactions depict the oxidizing beahviour of \(\mathrm{H}_2 \mathrm{SO}_4\)?
CorrectIncorrectHint
\(
2 \mathrm{HI}^{-1}+\stackrel{+6}{\mathrm{H}_2} \mathrm{SO}_4 \longrightarrow \mathrm{I}_2^0+\stackrel{+4}{\mathrm{SO}_2}+2 \mathrm{H}_2 \mathrm{O}
\)
In This reaction oxidation number of \(\mathrm{S}\) is decreasing from +6 to +4 hence undergoing reduction and for \(\mathrm{HI}\) oxidation Number of \(I\) is increasing from -1 to 0 hence undergoing oxidation therefore \(\mathrm{H}_2 \mathrm{SO}_4\) is acting as oxidising agent. -
Question 6 of 50
6. Question
Which of the following statements are correct concerning redox properties?
(i) A metal \(\mathrm{M}\) for which \(E^{\ominus}\) for the half life reaction \(\mathrm{M}^{\mathrm{n}+}+\mathrm{ne}^{-} \rightleftharpoons \mathrm{M}\) is very negative will be a good reducing agent.
(ii) The oxidizing power of the halogens decreases from chlorine to iodine.
(iii) The reducing power of hydrogen halides increases from hydrogen chloride to hydrogen iodideCorrectIncorrectHint
(i) \(\mathrm{Mn}^{\mathrm{n}+}+\mathrm{ne}^{-} \rightleftharpoons \mathrm{M}\), for this reaction, high negative value of \(E^{\ominus}\) indicates lower reduction potential, that means \(\mathrm{M}\) will be a good reducing agent.
\(
\left[\begin{array}{c}
\text { Stronger reducing agent } \Rightarrow \text { Easy to oxidise } \\
\Downarrow \\
\text { Lower reduction potential } \Leftarrow \text { higher oxidation potential }
\end{array}\right]
\)
\(
\begin{array}{lllll}
\text { Element } & & & & & & & \mathrm{F} & & \mathrm{C} 1 & & \mathrm{Br} & &\mathrm{I}
\end{array}
\)
\(
\text { Reduction potential } E^{\ominus} +2.87+1.36+1.06+0.54
\)
As reduction potential decreases from fluorine to iodine, oxidising nature also decreases from fluorine to iodine.
(iii) The size of halide ions increases from \(\mathrm{F}^{-}\)to \(\mathrm{I}^{-}\). The bigger ion can loose electron easily. Hence the reducing nature increases from \(\mathrm{HF}\) to \(\mathrm{HI}\). -
Question 7 of 50
7. Question
In the following balanced reaction,
\(
X \mathrm{MnO}_4^{-}+Y{\mathrm{C}_2} \mathrm{O}_4^{2-}+Z \mathrm{H}^{+} \rightleftharpoons X \mathrm{Mn}^{2+}+2 \mathrm{YCO}_2+\frac{Z}{2} \mathrm{H}_2 \mathrm{O}
\)
values of \(X, Y\) and \(Z\) respectively areCorrectIncorrectHint
\(
X \mathrm{MnO}_4^{-}+Y{\mathrm{C}_2} \mathrm{O}_4^{2-}+Z \mathrm{H}^{+} \rightleftharpoons X \mathrm{Mn}^{2+}+2 \mathrm{YCO}_2+\frac{Z}{2} \mathrm{H}_2 \mathrm{O}
\)
First half reaction
\(
\mathrm{MnO}_4^{-} \longrightarrow \mathrm{Mn}^{++} \dots(i)
\)
On balancing
\(
\mathrm{MnO}_4^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \dots(ii)
\)
\(
\text { Second } \mathrm{h}
\)
\(
\mathrm{C}_2 \mathrm{O}_4^{–} \longrightarrow 2 \mathrm{CO}_2 \dots(iii)
\)
On balancing
\(
\mathrm{C}_2 \mathrm{O}_4^{–} \longrightarrow 2 \mathrm{CO}_2+2 \mathrm{e}^{-} \dots(iv)
\)
On multiplying eqn. (ii) by 5 and (iv) by 2 and then adding we get
\(
2 \mathrm{MnO}_4^{-}+5 \mathrm{C}_2 \mathrm{O}_4^{–}+16 \mathrm{H}^{+} \longrightarrow 2 \mathrm{Mn}^{++}+10 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}
\)The above reaction is the balanced chemical reaction. Thus the value of \(x, y\), and \(\mathrm{z}\) is 2,5 , and 16 .
-
Question 8 of 50
8. Question
Arrange the following in the order of their decreasing electrode potentials: \(\mathrm{Mg}, \mathrm{K}, \mathrm{Ba}\) and \(\mathrm{Ca}\)
CorrectIncorrectHint
Order of decreasing electrode potentials of \(\mathrm{Mg}, \mathrm{K}, \mathrm{Ba}\) and \(\mathrm{Ca}\) is
\(
\mathrm{Mg}>\mathrm{Ca}>\mathrm{Ba}>\mathrm{K}
\)
It can be explained by their standard reduction potentials.
\(
E_{K+\mid K}^{\ominus}=-2.925
\)
\(
E_{B a^2+\mid B a}^{\ominus}=-2.90
\)
\(
E_{\mathrm{Ca}^{2+} \mid \mathrm{Ca}}^{\ominus}=-2.87
\)
\(
E_{M g^{2+} \mid M g}^{\ominus}=-2.37
\)
Highly negative value of \(\mathrm{E}_{\mathrm{red}}^{\ominus}\) shows the least value of electrode potential. -
Question 9 of 50
9. Question
Given
\(
\mathrm{XNa}_2 \mathrm{HAsO}_3+\mathrm{YNaBrO}_3+\mathrm{ZHCl} \rightarrow \mathrm{NaBr}+\mathrm{H}_3 \mathrm{AsO}_4+\mathrm{NaCl}
\)
The values of \(\mathrm{X}, \mathrm{Y}\) and \(\mathrm{Z}\) in the above redox reaction are respectively :CorrectIncorrectHint
(c) On balancing the given reaction, we find
\(
3 \mathrm{Na}_2 \mathrm{HAsO}_3+\mathrm{NaBrO}_3+6 \mathrm{HCl}\longrightarrow 6 \mathrm{NaCl}+3 \mathrm{H}_3 \mathrm{AsO}_4+\mathrm{NaBr}
\) -
Question 10 of 50
10. Question
Oxidation state of sulphur in anions \(\mathrm{SO}_3^{2-}, \mathrm{S}_2 \mathrm{O}_4^{2-}\) and \(\mathrm{S}_2 \mathrm{O}_6^{2-}\) increases in the orders :
CorrectIncorrectHint
(c)
\(
\begin{aligned}
& \text { In } \mathrm{SO}_3^{–} \\
& x+3(-2)=-2 ; x=+4 \\
& \text { In } \mathrm{S}_2 \mathrm{O}_4^{–} \\
& 2 x+4(-2)=-2 \\
& 2 x-8=-2 \\
& 2 x=6 ; \quad x=+3 \\
& \text { In } \mathrm{S}_2 \mathrm{O}_6^{2-} \\
& 2 x+6(-2)=-2 \\
& 2 x=10 ; \quad x=+5
\end{aligned}
\)
hence the correct order is
\(
\mathrm{S}_2 \mathrm{O}_4^{–}<\mathrm{SO}_3^{–}<\mathrm{S}_2 \mathrm{O}_6^{–}
\) -
Question 11 of 50
11. Question
Amongst the following, identify the species with an atom in +6 oxidation state:
CorrectIncorrectHint
(d) \(\mathrm{CrO}_2 \mathrm{Cl}_2\)
Let O. No. of \(\mathrm{Cr}=x\)
\(
\begin{aligned}
\therefore \quad & x+2(-2)+2(-1)=0 \\
& x-4-2=0 \\
\therefore \quad & x=+6
\end{aligned}
\) -
Question 12 of 50
12. Question
Which one of the following cannot function as an oxidising agent?
CorrectIncorrectHint
(a) If an electronegative element is in its lowest possible oxidation state in a compound or in free state. It can function as a powerful reducing agent.
e.g. \(\mathrm{I}^{-}\) -
Question 13 of 50
13. Question
A compound of \(\mathrm{Xe}\) and \(\mathrm{F}\) is found to have \(53.5 \%\) of \(\mathrm{Xe}\). What is oxidation number of \(\mathrm{Xe}\) in this compound?
CorrectIncorrectHint
\(
X e=53.5 \% \therefore F=46.5 \%
\)
Relative number of atoms \(\mathrm{Xe}\)
\(
=\frac{53.5}{131.2}=0.4 \text { and } \mathrm{F}=\frac{46.5}{19}=2.4
\)
Simple ratio \(\mathrm{Xe}=1\) and \(\mathrm{F}=6\); Molecular formula is \(\mathrm{XeF}_6\) -
Question 14 of 50
14. Question
Copper becomes green when exposed to moist air for a long period. This is due to:
CorrectIncorrectHint
When a copper vessel is exposed to moist air for a long time it develops a green layer on its surface. Copper corrodes by oxidation in which it reacts with oxygen in the air to form copper oxide.
Copper oxide then combines with carbon dioxide to make copper carbonate, which gives it a green colour. This process is called corrosion of copper.
The green material is a mixture of copper hydroxide \(\left(\mathrm{Cu}(\mathrm{OH})_2\right)\) and copper carbonate \(\left(\mathrm{CuCO}_3\right)\). The following is the reaction:
\(
2 \mathrm{Cu}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{g})+\mathrm{CO}_2+\mathrm{O}_2 \rightarrow \mathrm{Cu}(\mathrm{OH})_2+\mathrm{CuCO}_3(\mathrm{~s})
\)Copper(II) carbonate is a blue-green compound.
-
Question 15 of 50
15. Question
In the standardization of \(\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3\) using \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\) by iodometry, the equivalent weight of \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\) is
CorrectIncorrectHint
In iodometry, \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\) liberates \(\mathrm{I}_2\) from iodides ( \(\mathrm{NaI}\) or \(\mathrm{KI})\). Which is titrated with \(\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3\) solution.
\(
\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+\mathrm{I}^{-}+\mathrm{H}^{+} \longrightarrow \mathrm{Cr}^{3+}+\mathrm{I}_2
\)
Here, one mole of \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\) accepts 6 mole of electrons.
\(
\therefore \text { Equivalent weight }=\frac{\text { molecular weight }}{6}
\) -
Question 16 of 50
16. Question
Consider the reactions:
\(
\begin{aligned}
& \mathrm{H}_2 \mathrm{SO}_3(\mathrm{aq})+\mathrm{Sn}^{4+}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \quad \rightarrow \mathrm{Sn}^{2+}(\mathrm{aq})+\mathrm{HSO}_4^{-}(\mathrm{aq})+3 \mathrm{H}^{+}(\mathrm{aq})
\end{aligned}
\)
Which of the following statements is correct?CorrectIncorrectHint
\(
\mathrm{H}_2 \stackrel{+4}{\mathrm{SO}_3}(\mathrm{aq})+\mathrm{Sn}^{4+}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(1) \longrightarrow \mathrm{Sn}^{2+}(\mathrm{aq})+\stackrel{+6}{\mathrm{HSO}_4^{-}}(\mathrm{aq})+3 \mathrm{H}^{+}
\)
Hence \(\mathrm{H}_2 \mathrm{SO}_3\) is the reducing agent because it undergoes oxidation. -
Question 17 of 50
17. Question
The species that undergoes disproportionation in an alkaline medium are
CorrectIncorrectHint
\(
\mathrm{Cl}_2^0+2 \mathrm{NaOH} \rightarrow \stackrel{-1}{\mathrm{NaCl}}+\stackrel{+1}{\mathrm{NaClO}}+\mathrm{H}_2 \mathrm{O}
\)
\(
\stackrel{+6}{\mathrm{3M}} \mathrm{nO}_4^{–}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \stackrel{+7}{\mathrm{MnO}_4^{-}}+\stackrel{+4}{\mathrm{M}} \mathrm{nO}_2+4 \mathrm{OH}^{-}
\)
\(
\stackrel{+4}{\mathrm{2NO}_2}+\mathrm{H}_2 \mathrm{O} \rightarrow \stackrel{+5}{\mathrm{HNO}_3}+\stackrel{+3}{\mathrm{HNO}_2}
\)
All undergo disproportionation -
Question 18 of 50
18. Question
How many electrons are involved in the following redox reaction?
\(
\mathrm{Cr}_2 \mathrm{O}_7^{2-}+\mathrm{Fe}^{2+}+\mathrm{C}_2 \mathrm{O}_4^{2-} \rightarrow \mathrm{Cr}^{3+}+\mathrm{Fe}^{3+}+\mathrm{CO}_2 \text { (Unbalanced) }
\)CorrectIncorrectHint
The reaction given is
\(
\mathrm{Cr}_2 \mathrm{O}_7^{2-}+\mathrm{Fe}^{2+}+\mathrm{C}_2 \mathrm{O}_4^{2-} \longrightarrow \mathrm{Cr}^{3+}+\mathrm{Fe}^{3+}+\mathrm{CO}_2\)
\(
\mathrm{Cr}_2 \mathrm{O}_7{ }^{2-} \longrightarrow 2 \mathrm{Cr}^{3+}
\)
On balancing
\(
14 \mathrm{H}^{+}+\mathrm{Cr}_2 \mathrm{O}_7^{2-}+6 \mathrm{e}^{-} \longrightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O} \dots(i)
\)
\(
\mathrm{Fe}^{2+} \longrightarrow \mathrm{Fe}^{3+}+\mathrm{e}^{-} \dots(ii)
\)
\(
\mathrm{C}_2 \mathrm{O}_4{ }^{2-} \longrightarrow 2 \mathrm{CO}_2+2 \mathrm{e}^{-} \dots(iii)
\)
On adding all the three equations.
\(
\mathrm{Cr}_2 \mathrm{O}_7^{2-}+\mathrm{Fe}^{2+}+\mathrm{C}_2 \mathrm{O}_4^{2-}+14 \mathrm{H}^{+}+3 \mathrm{e}^{-}
\)
\(
\longrightarrow 2 \mathrm{Cr}^{3+}+\mathrm{Fe}^{3+}+2 \mathrm{CO}_2+7 \mathrm{H}_2 \mathrm{O}
\)Hence the total no. of electrons involved in the reaction =3
-
Question 19 of 50
19. Question
\(\mathrm{H}_2 \mathrm{~S}\) acts only as a reducing agent while \(\mathrm{SO}_2\) can act both as a reducing and oxidizing agent because
CorrectIncorrectHint
\(\mathrm{H}_2 \mathrm{~S}\), the oxidation state of \(\mathrm{S}\) is -2 . So it cannot accept more electrons because on accepting 2 electrons \(S\) accquires a noble gas configuration. So, it can acts only as a reducing agent by loosing electron. On the other hand, the oxidation state of \(\mathrm{S}\) in \(\mathrm{SO}_2\) is +4 which is an intermediate oxidation state of sulphur so it can reduce as well oxidise.
-
Question 20 of 50
20. Question
Which of the following cannot act as reducing agent?
CorrectIncorrectHint
In all the given compounds oxidation number of non metal is +4 . As \(\mathrm{C}\) belongs to group IV and it is in its maximum oxidation state. So, reduction in oxidation number of nonmetal is not possible only in \(\mathrm{CO}_2\). As we know that reduction is always accompanied by an increase in oxidation number of reducing agent. So, \(\mathrm{CO}_2\) cannot acts as reducing agent among the given choices.
-
Question 21 of 50
21. Question
\(
\mathrm{C}_2 \mathrm{H}_6(\mathrm{~g})+\mathrm{nO}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})
\)
In this equation, the ratio of the coefficients of \(\mathrm{CO}_2\) and \(\mathrm{H}_2 \mathrm{O}\) isCorrectIncorrectHint
The balanced equation is
\(
2 \mathrm{C}_2 \mathrm{H}_6+7 \mathrm{O}_2 \rightarrow 4 \mathrm{CO}_2+6 \mathrm{H}_2 \mathrm{O} \text {. }
\)Ratio of the coefficients of \(\mathrm{CO}_2\) and \(\mathrm{H}_2 \mathrm{O}\) is \(4: 6\) or \(2: 3\).
-
Question 22 of 50
22. Question
Which substance serves as reducing agent in the following reaction?
\(
14 \mathrm{H}^{+}+\mathrm{Cr}_2 \mathrm{O}_7^{2-}+3 \mathrm{Ni} \longrightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O}+3 \mathrm{Ni}^{2+}
\)CorrectIncorrectHint
The compound undergo oxidation itself and reduces others is known as reducing agent. In this reaction \(\mathrm{O}\). \(\mathrm{N}\). of \(\mathrm{Ni}\) changes from 0 to +2 and hence \(\mathrm{Ni}\) acts as a reducing agent.
-
Question 23 of 50
23. Question
Which of the following reactions depict the oxidising behaviour of \(\mathrm{H}_2 \mathrm{SO}_4\) :
CorrectIncorrectHint
\(
\begin{aligned}
& \stackrel{-1}{\mathrm{2HI}}+\quad \stackrel{+6}{\mathrm{H}_2} \mathrm{SO}_4 \rightarrow \stackrel{0}{\mathrm{I}}+\stackrel{+4}{\mathrm{SO}_2}+2 \mathrm{H}_2 \mathrm{O} \\
& \text { Oxidised Reduced } \\
& \text { RA } \quad \quad { O A } \\
&
\end{aligned}
\) -
Question 24 of 50
24. Question
Which one of the following reactions involves disproportionation?
CorrectIncorrectHint
A reaction, in which a substance undergoes simultaneous oxidation and reduction, is called disproportionation reaction. In these reactions, the same substance simultaneously acts as an oxidising agent and as a reducing agent. Here \(\mathrm{Cl}\) undergoes simultaneous oxidation and reduction.
\(
2 \mathrm{KOH}+\mathrm{Cl}_2 \rightarrow \mathrm{KCl}+\mathrm{KOCl}+\mathrm{H}_2 \mathrm{O} .
\)
\(
\begin{array}{lll}
& & & & 0 & & -1 & & +1
\end{array}
\) -
Question 25 of 50
25. Question
Point out the correct statement of the following about \(\mathrm{Na}_2 \mathrm{~S}_4 \mathrm{O}_6\).
CorrectIncorrectHint
\(\mathrm{Na}_2 \mathrm{~S}_4 \mathrm{O}_6\) has the structure:
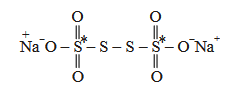
O.N. of two \(\mathrm{S}^*\) atoms are +5 each and that of other two \(\mathrm{S}\) atoms is zero each. -
Question 26 of 50
26. Question
The pair of compounds in which both the metals are in the highest possible oxidation state is
CorrectIncorrectHint
\(\mathrm{CrO}_2 \mathrm{Cl}_2, \mathrm{MnO}_4^{-} ; \mathrm{O} . \mathrm{N}\). of \(\mathrm{Cr}\) and \(\mathrm{Mn}\) are +6 and +7 respectively.
-
Question 27 of 50
27. Question
Thiosulphate reacts differently with iodine and bromine in the reactions given below.
\(
\begin{aligned}
2 \mathrm{~S}_2 \mathrm{O}_3^{2-}+\mathrm{I}_2 & \rightarrow \mathrm{S}_4 \mathrm{O}_6^{2-}+2 \mathrm{I}^{-} \\
\mathrm{S}_2 \mathrm{O}_3^{2-}+2 \mathrm{Br}_2+5 \mathrm{H}_2 \mathrm{O} & \rightarrow 2 \mathrm{SO}_4^{2-}+2 \mathrm{Br}^{-}+10 \mathrm{H}^{+}
\end{aligned}
\)
Which of the following statements justifies the above dual behaviour of thiosulphate?CorrectIncorrectHint
\(
\stackrel{+2}{\mathrm{2S}_2 \mathrm{O}_3^{2-}}(\mathrm{aq})+\stackrel{0}{\mathrm{I}_2}(\mathrm{~s}) \rightarrow \stackrel{2.5}{\mathrm{~S}_4 \mathrm{O}_6^{2-}}(\mathrm{aq})+2 \mathrm{I}^{-}(\mathrm{aq})
\)
\(
\stackrel{+2}{\mathrm{S}_2} \mathrm{O}_3^{2-}(\mathrm{aq})+2 \stackrel{0}{\mathrm{Br}_2}(l)+5 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \stackrel{+6}{\mathrm{2S} \mathrm{O}_4^{2-}}(\mathrm{aq})+4 \mathrm{Br}^{-}(\mathrm{aq})+10 \mathrm{H}^{+}(\mathrm{aq})
\)
Hence, bromine is a stronger oxidising agent than \(I_2\), as it oxidises \(\mathrm{S}\) of \(\mathrm{S}_2 \mathrm{O}_3^{2-}\) to \(\mathrm{SO}_4^{2-}\) whereas \(\mathrm{I}_2\) oxidises it only into \(\mathrm{S}_4 \mathrm{O}_6^{2-}\) ion. -
Question 28 of 50
28. Question
Standard reduction potentials of the half reactions are given below :
\(
\begin{aligned}
& \mathrm{F}_2(\mathrm{~g})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{~F}^{-}(\mathrm{aq}) ; E^{\ominus}=+2.85 \mathrm{~V} \\
& \mathrm{Cl}_2(\mathrm{~g})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cl}^{-}(\mathrm{aq}) ; E^{\ominus}=+1.36 \mathrm{~V} \\
& \mathrm{Br}_2(\mathrm{l})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Br}^{-}(\mathrm{aq}) ; E^{\ominus}=+1.06 \mathrm{~V} \\
& \mathrm{I}_2(\mathrm{~s})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{I}^{-}(\mathrm{aq}) ; E^{\ominus}=+0.53 \mathrm{~V}
\end{aligned}
\)
The strongest oxidising and reducing agents respectively are:CorrectIncorrectHint
Higher the value of reduction potential higher will be the oxidising power whereas the lower the value of reduction potential higher will be the reducing power.
-
Question 29 of 50
29. Question
A mixture of potassium chlorate, oxalic acid and sulphuric acid is heated. During the reaction which element undergoes maximum change in the oxidation number?
CorrectIncorrectHint
\(
\stackrel{+5}{\mathrm{KClO}_3}+\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4+\stackrel{+6}{\mathrm{H}_2 \mathrm{SO}_4} \rightarrow \stackrel{+6}{\mathrm{~K}_2 \mathrm{SO}_4}+\stackrel{-1}{\mathrm{~KCl}}+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}
\)
i.e. maximum change in oxidation number is observed in \(\mathrm{Cl}(+5[latex] to -1\))[/latex] -
Question 30 of 50
30. Question
\(\mathrm{Zn}\) gives \(\mathrm{H}_2\) gas with \(\mathrm{H}_2 \mathrm{SO}_4\) and \(\mathrm{HCl}\) but not with \(\mathrm{HNO}_3\) because
CorrectIncorrectHint
Zinc gives \(\mathrm{H}_2\) gas with dil \(\mathrm{H}_2 \mathrm{SO}_4 / \mathrm{HCl}\) but not with \(\mathrm{HNO}_3\) because in \(\mathrm{HNO}_3, \mathrm{NO}_3{ }^{-}\)ion is reduced and give \(\mathrm{NH}_4 \mathrm{NO}_3\), \(\mathrm{N}_2 \mathrm{O}\), \(\mathrm{NO}\) and \(\mathrm{NO}_2\) (based upon the concentration of \(\left.\mathrm{HNO}_3\right)\)
\(
\left[\mathrm{Zn}+\underset{\text { (nearly } 6 \%)}{2 \mathrm{HNO}_3} \longrightarrow \mathrm{Zn}\left(\mathrm{NO}_3\right)_2+2 \mathrm{H}\right] \times 4
\)
\(
\begin{aligned}
& \mathrm{HNO}_3+8 \mathrm{H} \longrightarrow \mathrm{NH}_3+3 \mathrm{H}_2 \mathrm{O} \\
& \mathrm{NH}_3+\mathrm{HNO}_3 \longrightarrow \mathrm{NH}_4 \mathrm{NO}_3
\end{aligned}
\)
\(
4 \mathrm{Zn}+10 \mathrm{HNO}_3 \longrightarrow 4 \mathrm{Zn}\left(\mathrm{NO}_3\right)_2+\mathrm{NH}_4 \mathrm{NO}_3+3 \mathrm{H}_2 \mathrm{O}
\)
\(\mathrm{Zn}\) is on the top position of hydrogen in electrochemical series. So \(\mathrm{Zn}\) displaces \(\mathrm{H}_2\) from dilute \(\mathrm{H}_2 \mathrm{SO}_4\) and \(\mathrm{HCl}+\mathrm{H}_2\) -
Question 31 of 50
31. Question
\(5 \mathrm{~g}\) of \(\mathrm{NaOH}\) was dissolved in deionized water to prepare a \(450 \mathrm{~mL}\) stock solution. What volume (in \(\mathrm{mL})\) of this solution would be required to prepare \(500 \mathrm{~mL}\) of \(0.1 \mathrm{M}\) solution ?
Given : Molar Mass of \(\mathrm{Na}, \mathrm{O}\) and \(\mathrm{H}\) is 23,16 and 1 \(\mathrm{g} \mathrm{mol}^{-1}\) respectivelyCorrectIncorrectHint
\(
\begin{aligned}
& M=\frac{5}{40} \times \frac{1000}{450} \\
& M_1 V_1=M_2 V_2 \\
& \left(\frac{5}{40} \times \frac{1000}{450}\right) \times V_1=0.1 \times 500 \\
& V_1=180
\end{aligned}
\) -
Question 32 of 50
32. Question
Which will undergo deprotonation most readily in basic medium?
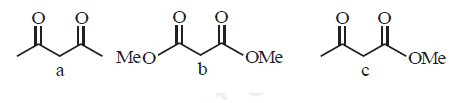 CorrectIncorrect
CorrectIncorrectHint
Most easily deprotonation
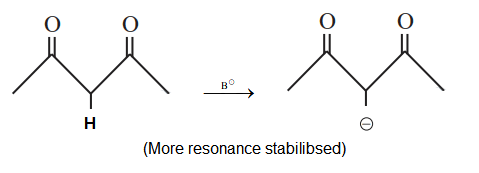
-
Question 33 of 50
33. Question
The density of a monobasic strong acid (Molar mass \(24.2 \mathrm{~g} \mathrm{~mol}\) ) is \(1.21 \mathrm{~kg} \mathrm{~L}\). The volume of its solution required for the complete neutralization of \(25 \mathrm{~mL}\) of \(0.24 \mathrm{M} \mathrm{NaOH}\) is ______. \(10^{-2} \mathrm{~mL}\) (Nearest integer)
CorrectIncorrectHint
millimole of \(\mathrm{NaOH}=0.24 \times 25\)
\(\begin{aligned} & \therefore \quad \text { millimole of acid }=0.24 \times 25 \\ & \Rightarrow \quad \text { mass of acid }=0.24 \times 25 \times 24.2 \mathrm{mg}\end{aligned}\)
for pure acid,
\(
\mathrm{V}=\frac{\mathrm{W}}{\mathrm{d}} ;(\mathrm{d}=1.21 \mathrm{~kg} / \mathrm{L}=1.21 \mathrm{~g} / \mathrm{ml})
\)
\(
\begin{aligned}
\therefore \mathrm{V}= & \frac{0.24 \times 25 \times 24.2}{1.12} \times 10^{-3} \\
& =120 \times 10^{-3} \mathrm{ml} \\
& =12 \times 10^{-2} \mathrm{ml}
\end{aligned}
\) -
Question 34 of 50
34. Question
An indicator ‘ \(\mathrm{X}\) ‘ is used for studying the effect of variation in concentration of iodide on the rate of reaction of iodide ion with \(\mathrm{H}_2 \mathrm{O}_2\) at room temp. The indicator ‘ \(\mathrm{X}\) ‘ forms blue colored complex with compound ‘ \(A\) ‘ present in the solution. The indicator ‘ \(\mathrm{X}\) ‘ and compound ‘ \(\mathrm{A}\) ‘ respectively are
CorrectIncorrectHint
\(
\mathrm{I}^{-}+\mathrm{H}_2 \mathrm{O}_2 \longrightarrow \underset{\text { (A) }}{\mathrm{I}_2}+\mathrm{H}_2 \mathrm{O}
\)
\(
\mathrm{I}_2+\underset{\text { (Indicator) }}{\mathrm{Starch}} \longrightarrow \text { Blue }
\) -
Question 35 of 50
35. Question
The volume of \(\mathrm{HCl}\), containing \(73 \mathrm{~g} \mathrm{~L}^{-1}\), required to completely neutralise \(\mathrm{NaOH}\) obtained by reacting \(0.69 \mathrm{~g}\) of metallic sodium with water, is _____ \(\mathrm{mL}\). (Nearest Integer)
(Given : molar Masses of \(\mathrm{Na}, \mathrm{Cl}, \mathrm{O}, \mathrm{H}\) are 23, \(35.5,16\) and \(1 \mathrm{~g} \mathrm{~mol}^{-1}\) respectively)CorrectIncorrectHint
Mole of \(\mathrm{Na}=\frac{0.69}{23}=3 \times 10^{-2}\)
\(
\mathrm{Na}+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{NaOH}+\frac{1}{2} \mathrm{H}_2
\)
By using POAC
Moles of \(\mathrm{NaOH}=3 \times 10^{-2}\)
\(\mathrm{NaOH}\) reacts with \(\mathrm{HCl}\)
No. of equivalent of \(\mathrm{NaOH}=\mathrm{No}\). of equivalent of \(\mathrm{HCl}\)
\(
3 \times 10^{-2} \times 1=\frac{73}{36.5} \times \mathrm{V}(\text { in } \mathrm{L}) \times 1
\)
\(
\mathrm{V}=1.5 \times 10^{-2} \mathrm{~L}
\)
Volume of \(\mathrm{HCl}=15 \mathrm{ml}\). -
Question 36 of 50
36. Question
The number of electrons involved in the reduction of permanganate to manganese dioxide in acidic medium is
CorrectIncorrectHint
The number of electrons involved in the reduction of permanganate to manganese dioxide in acidic medium is 3 .
\(
\stackrel{+7}{\mathrm{MnO}_4^{-}}+4 \mathrm{H}^{+}+3 \mathrm{e}^{-} \longrightarrow \stackrel{+4}{\mathrm{Mn}} \mathrm{{O}_2}+2 \mathrm{H}_2 \mathrm{O}
\) -
Question 37 of 50
37. Question
\(\mathrm{KMnO}_4\) oxidises \(\mathrm{I}\) in acidic and neutral/faintly alkaline solution, respectively to
CorrectIncorrectHint
In acidic medium
\(
2 \mathrm{MnO}_4^{-}+10 \mathrm{I}^{-}+16 \mathrm{H}^{+} \rightarrow 2 \mathrm{Mn}^{2+}+5 \mathrm{I}_2+8 \mathrm{H}_2 \mathrm{O}
\)
In neutral/faintly alkaline solution
\(
2 \mathrm{MnO}_4^{-}+\mathrm{I}^{-}+\mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{MnO}_2+2 \mathrm{OH}^{-}+\mathrm{IO}_3^{-}
\) -
Question 38 of 50
38. Question
\(25 \mathrm{~mL}\) of an aqueous solution of \(\mathrm{KCl}\) was found to require \(20 \mathrm{~mL}\) of \(1 \mathrm{M} \mathrm{~AgNO}_3\) solution when titrated using \(\mathrm{K}_2 \mathrm{CrO}_4\) as an indicator. What is the depression in freezing point of \(\mathrm{KCl}\) solution of the given concentration?
(Nearest integer).
(Given : \(\mathrm{K}_{\mathrm{f}}=2.0 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}\) )
Assume
1) \(100 \%\) ionization and
2) density of the aqueous solution as \(1 \mathrm{~g} \mathrm{~mL}^{-1}\)CorrectIncorrectHint
At equivalence point, mmole of \(\mathrm{KCl}=\) mmole of \(\mathrm{AgNO}_3=20 \mathrm{~mmole}\)
Volume of solution \(=25 \mathrm{~ml}\)
Mass of solution \(=25 \mathrm{~gm}\)
Mass of solvent
\(
\begin{aligned}
& =25-\text { mass of solute } \\
& =25-\left[20 \times 10^{-3} \times 74.5\right] \\
& =23.51 \mathrm{~gm}
\end{aligned}
\)
\(
\begin{aligned}
& \text { Molality of } \mathrm{KCl}=\frac{\text { mole of } \mathrm{KCl}}{\text { mass of solvent in } \mathrm{kg}} \\
& =\frac{20 \times 10^{-3}}{23.51 \times 10^{-3}}=0.85 \\
& \mathrm{i} \text { of } \mathrm{KCl}=2(100 \% \text { ionisation }) \\
& \Delta \mathrm{T}_{\mathrm{f}}=\mathrm{i} \times \mathrm{K}_{\mathrm{f}} \times \mathrm{m} \\
& =2 \times 2 \times 0.85 \\
& =3.4 \\
& \simeq 3
\end{aligned}
\) -
Question 39 of 50
39. Question
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : An aqueous solution of \(\mathrm{KOH}\) when for volumetric analysis, its concentration should be checked before the use.
Reason (R) : On aging, \(\mathrm{KOH}\) solution absorbs atmospheric \(\mathrm{CO}_2\).
In the light of the above statements, choose the correct answer from the options given below.CorrectIncorrectHint
\(\mathrm{KOH}\) absorb \(\mathrm{CO}_2\)
So its concentration should be checked. -
Question 40 of 50
40. Question
\(\mathrm{SO}_2 \mathrm{Cl}_2\) on reaction with excess of water results into acidic mixture
\(
\mathrm{SO}_2 \mathrm{Cl}_2+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{SO}_4+2 \mathrm{HCl}
\)
16 moles of \(\mathrm{NaOH}\) is required for the complete neutralisation of the resultant acidic mixture. The number of moles of \(\mathrm{SO}_2 \mathrm{Cl}_2\) used is:CorrectIncorrectHint
\(
\text { Let } \mathrm{n}\left(\mathrm{SO}_2 \mathrm{Cl}_2\right)=\mathrm{x} \text { moles }
\)
\(
\therefore \mathrm{n}\left(\mathrm{H}_2 \mathrm{SO}_4\right)=\mathrm{x}, \mathrm{n}(\mathrm{HCl})=2 \mathrm{x}
\)
\(
\Rightarrow \mathrm{n}\left(\mathrm{H}^{+}\right)=4 \mathrm{x}
\)
For Neutralisation
\(
\begin{aligned}
& \Rightarrow \mathrm{n}\left(\mathrm{H}^{+}\right)=\mathrm{n}\left(\mathrm{OH}^{-}\right) \\
& \Rightarrow 4 \mathrm{x}=16 \\
& \Rightarrow \mathrm{x}=4
\end{aligned}
\) -
Question 41 of 50
41. Question
In base vs. Acid titration, at the end point methyl orange is present as
CorrectIncorrectHint
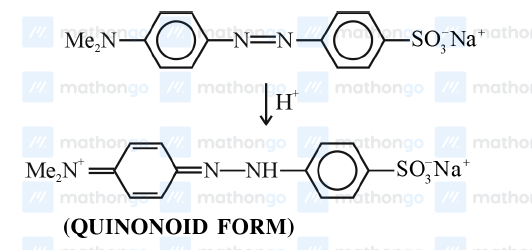
-
Question 42 of 50
42. Question
Which of the given reactions is not an example of disproportionation reaction?
CorrectIncorrectHint
\(
\stackrel{-1}{2 \mathrm{H}_2 \mathrm{O}_2} \longrightarrow \stackrel{2-}{2 \mathrm{H}_2 \mathrm{O}}+\stackrel{0}{\mathrm{O}_2} \text { : Disproportionation }
\)
\(
\stackrel{+4}{2 \mathrm{NO}_2}+\mathrm{H}_2 \mathrm{O} \rightarrow \stackrel{+5}{\mathrm{HNO}_3}+\stackrel{+3}{\mathrm{HNO}_2}: \text { Disproportionation }
\)
\(
\mathrm{MnO}_4^{-}+4 \mathrm{H}^{+}+3 \mathrm{e}^{-} \rightarrow \mathrm{MnO}_2+2 \mathrm{H}_2 \mathrm{O} \text { : reduction }
\)
\(
\stackrel{+6}{3 \mathrm{MnO}_4^{2-}}+4 \mathrm{H}^{+} \rightarrow 2 \stackrel{+7}{\mathrm{MnO}_4^{-}}+\stackrel{+4}{\mathrm{MnO}_2}+2 \mathrm{H}_2 \mathrm{O}: \text { Disproportionation }
\) -
Question 43 of 50
43. Question
The dark purple colour of \(\mathrm{KMnO}_4\) disappears in the titration with oxalic acid in acidic medium. The overall change in the oxidation number of manganese in the reaction is :
CorrectIncorrectHint
In acidic medium,
\(
\mathrm{MnO}_4^{-} \rightarrow \mathrm{Mn}^{+2}
\)
change in ox. no. \(=5\) -
Question 44 of 50
44. Question
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R. Assertion A : Phenolphthalein is a \(\mathrm{pH}\) dependent indicator, remains colourless in acidic solution and gives pink colour in basic medium
Reason \(\mathbf{R}\) : Phenolphthalein is a weak acid. It doesn’t dissociate in basic medium.
In the light of the above statements, choose the most appropriate answer from the options given below :CorrectIncorrectHint
Phenolphthalein dissociate in basic medium
\(
\mathrm{HPh}(\mathrm{aq}) \rightleftharpoons \mathrm{H}^{+}+\mathrm{Ph}^{-}
\)
\(
\text { (colourless) (Pink) }
\) -
Question 45 of 50
45. Question
\(20 \mathrm{~mL}\) of \(0.02 \mathrm{M}\) hypo solution is used for the titration of \(10 \mathrm{~mL}\) of copper sulphate solution, in the presence of excess of \(\mathrm{KI}\)using starch as an indicator. The molarity of \(\mathrm{Cu}^{2+}\) is found to be _____ \(\times 10^{-2} \mathrm{M}\) [nearest integer]
Given : \(2 \mathrm{Cu}^{2+}+4 \mathrm{I}^{-} \rightarrow \mathrm{Cu}_2 \mathrm{I}_2+\mathrm{I}_2\)
\(
\mathrm{I}_2+2 \mathrm{~S}_2 \mathrm{O}_3{ }^{2-} \rightarrow 2 \mathrm{I}^{-}+\mathrm{S}_4 \mathrm{O}_6{ }^{2-}
\)CorrectIncorrectHint
\(
\mathrm{n}_{\text {eq. }} \text { of } \mathrm{I}_2=\mathrm{n}_{\mathrm{eq}} \text { of } \mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3=20 \times 0.002 \times 1
\)
\(
2 \times \mathrm{n}_{\mathrm{mol}} \text { of } \mathrm{I}_2=0.4
\)
\(
\mathrm{n}_{\mathrm{mol}} \text { of } \mathrm{I}_2=0.2 \mathrm{~m} \mathrm{~mol}
\)
\(
\mathrm{n}_{\mathrm{mol}} \text { of } \mathrm{Cu}^{+2}=0.2 \times 2 \times 10^{-3}
\)
\(
\left[\mathrm{Cu}^{+2}\right]=\frac{0.4 \times 10^{-3}}{10 \times 10^{-3}}=0.04=4 \times 10^{-2}
\) -
Question 46 of 50
46. Question
\(20 \mathrm{~mL}\) of \(0.02 \mathrm{M} \mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\) solution is used for the titration of \(10 \mathrm{~mL}\) of \(\mathrm{Fe}^{2+}\) solution in the acidic medium.
The molarity of \(\mathrm{Fe}^{2+}\) solution is ______ \(\times 10^{-2} \mathrm{M}\). (Nearest Integer)CorrectIncorrectHint
Eq. of \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7=\) Eq. of \(\mathrm{Fe}^{2+}\)
\(\Rightarrow\) (Molarity \(\times\) volume \(\times\) n.f) of \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7=\) \(\left(\right.\) molarity \(\times\) volume \(\times\) n.f) of \(\mathrm{Fe}^{2+}\)
\(
\Rightarrow 0.02 \times 20 \times 6=\mathrm{M} \times 10 \times 1
\)
\(
\Rightarrow \mathrm{M}=0.24
\)
\(\Rightarrow\) Molarity \(=24 \times 10^{-2}\) -
Question 47 of 50
47. Question
Given below are two statements : One is labelled as Assertion \(\mathbf{A}\) and the other is labelled as Reason \(\mathbf{R}\) Assertion A : Permanganate titrations are not performed in presence of hydrochloric acid.
Reason R : Chlorine is formed as a consequence of oxidation of hydrochloric acid.
In the light of the above statements, choose the correct answer from the options given belowCorrectIncorrectHint
\(
2 \mathrm{KMnO}_4+16 \mathrm{HCl} \rightarrow 2 \mathrm{MnCl}_2+2 \mathrm{KCl}+8 \mathrm{H}_2 \mathrm{O}+\mathrm{Cl}_2
\)
\(\mathrm{HCl}\) gets oxidised by \(\mathrm{KMnO}_4\) into \(\mathrm{Cl}_2\) -
Question 48 of 50
48. Question
\(2 \mathrm{~L}\) of \(0.2 \mathrm{~M} \mathrm{~H}_2 \mathrm{SO}_4\) is reacted with \(2 \mathrm{~L}\) of \(0.1 \mathrm{~M}\) \(\mathrm{~NaOH}\) solution, the molarity of the resulting product \(\mathrm{Na}_2 \mathrm{SO}_4\) in the solution is _____ millimolar. (Nearest integer).
CorrectIncorrectHint
\(
\mathrm{H}_2 \mathrm{SO}_4+2 \mathrm{NaOH} \rightarrow \mathrm{Na}_2 \mathrm{SO}_4+2 \mathrm{H}_2 \mathrm{O}
\)
\(
\begin{aligned}
& 0.4 \mathrm{~mol} \quad 0.2 \mathrm{~mol} \quad – \\
& 0.3 \mathrm{~mol} \quad-\quad \quad 0.1 \mathrm{~mol}
\end{aligned}
\)
Molarity of \(\mathrm{Na}_2 \mathrm{SO}_4\) is \(\frac{0.1}{4}=0.025 \mathrm{M}\) \(=25 \mathrm{mM}\) -
Question 49 of 50
49. Question
A compound ‘ \(\mathrm{X}\) ‘ is a weak acid and it exhibits colour change at \(\mathrm{pH}\) close to the equivalence point during neutralization of \(\mathrm{NaOH}\) with \(\mathrm{CH}_3 \mathrm{COOH}\). Compound ‘ \(\mathrm{X}\) ‘ exists in ionized form in basic medium. The compound ‘ \(\mathrm{X}\) ‘ is :
CorrectIncorrectHint
Phenolphthalein is weak acid give colour in basic medium.
-
Question 50 of 50
50. Question
What is the oxidation number of \(\mathrm{P}\) in \(\mathrm{H}_3 \mathrm{PO}_4\) ?
CorrectIncorrectHint
let the oxidation no. of \(\mathrm{P}\) in \(\mathrm{H}_3 \mathrm{PO}_4\), be \(x\).
\(+1 \quad x \quad -2\)
\(
\mathrm{H}_3 \quad \mathrm{~P} \quad \quad \mathrm{O}_4
\)
Calculate the sum of the oxidation numbers of all the atoms
\(
\begin{gathered}
3(+1)+x+4(-2)=0 \\
=3+x-8=x-5=0 \\
x=+5
\end{gathered}
\)