Classification of elements and Periodicity Quiz-1
Multiple Choice Questions with one Correct Answer
Quiz Summary
0 of 50 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 50 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 50
1. Question
1 point(s)The atomic number of the element unnilennium is :
CorrectIncorrect -
Question 2 of 50
2. Question
1 point(s)The group number, number of valence electrons, and valency of an element with atomic number 15 , respectively, are :
CorrectIncorrect -
Question 3 of 50
3. Question
1 point(s)The element with \(Z=120\) (not yet discovered) will be an/a:
CorrectIncorrect -
Question 4 of 50
4. Question
1 point(s)Similarity in chemical properties of the atoms of elements in a group of the periodic table is most closely related to:
CorrectIncorrect -
Question 5 of 50
5. Question
1 point(s)Which of the following has the maximum number of unpaired electrons?
CorrectIncorrect -
Question 6 of 50
6. Question
1 point(s)The statement that is not correct for the periodic classification of element is
CorrectIncorrect -
Question 7 of 50
7. Question
1 point(s)The atomic number of Unnilunium is _____?
CorrectIncorrect -
Question 8 of 50
8. Question
1 point(s)The set that contains atomic numbers of only transition elements, is :
CorrectIncorrect -
Question 9 of 50
9. Question
1 point(s)The correct order of the ionic radii of \(\mathrm{O}^{2-}, \mathrm{N}^{3-}, \mathrm{F}^{-}, \mathrm{Mg}^{2+}, \mathrm{Na}^{+}\)and \(\mathrm{Al}^{3+}\) is:
CorrectIncorrect -
Question 10 of 50
10. Question
1 point(s)The elements with atomic numbers 101 and 104 belong to, respectively:
CorrectIncorrect -
Question 11 of 50
11. Question
1 point(s)The process that is NOT endothermic in nature is :
CorrectIncorrect -
Question 12 of 50
12. Question
1 point(s)The ionic radii of \(\mathrm{O}^{2-}, \mathrm{F}^{-}, \mathrm{Na}^{+}\)and \(\mathrm{Mg}^{2+}\) are in the order:
CorrectIncorrect -
Question 13 of 50
13. Question
1 point(s)Among the statements (I – IV), the correct ones are :
(I) Be has smaller atomic radius compared to \(\mathrm{Mg}\).
(II) Be has higher ionization enthalpy than \(\mathrm{Al}\).
(III) Charge/radius ratio of \(\mathrm{Be}\) is greater than that of \(\mathrm{Al}\).
(IV) Both \(\mathrm{Be}\) and \(\mathrm{Al}\) form mainly covalent compounds.CorrectIncorrect -
Question 14 of 50
14. Question
1 point(s)The five successive ionization enthalpies of an element are 800,2427 , 3658,25024 and \(32824 \mathrm{~kJ} \mathrm{~mol}^{-1}\). The number of valence electrons in the element is :
CorrectIncorrect -
Question 15 of 50
15. Question
1 point(s)In general, the property (magnitudes only) that shows an opposite trend in comparison to other properties across a period is :
CorrectIncorrect -
Question 16 of 50
16. Question
1 point(s)Three elements \(\mathrm{X}, \mathrm{Y}\) and \(\mathrm{Z}\) are in the \(3^{\text {rd }}\) period of the periodic table. The oxides of \(\mathrm{X}, \mathrm{Y}\) and \(\mathrm{Z}\), respectively, are basic, amphoteric and acidic. The correct order of the atomic numbers of \(\mathrm{X}, \mathrm{Y}\) and \(\mathrm{Z}\) is :
CorrectIncorrect -
Question 17 of 50
17. Question
1 point(s)\(\mathrm{B}\) has a smaller first ionization enthalpy than \(\mathrm{Be}\). Consider the following statements:
(I) it is easier to remove \(2 p\) electron than \(2 s\) electron
(II) \(2 p\) electron of \(\mathrm{B}\) is more shielded from the nucleus by the inner core of electrons than the \(2 s\) electrons of \(\mathrm{Be}\)
(III) \(2 s\) electron has more penetration power than \(2 p\) electron
(IV) atomic radius of \(\mathrm{B}\) is more than \(\mathrm{Be}\) (atomic number \(\mathrm{B}=5, \mathrm{Be}=4\) )
The correct statements are:CorrectIncorrect -
Question 18 of 50
18. Question
1 point(s)The acidic, basic and amphoteric oxides, respectively, are:
CorrectIncorrect -
Question 19 of 50
19. Question
1 point(s)The first and second ionisation enthalpies of a metal are 496 and 4560 \(\mathrm{kJ} \mathrm{mol}{ }^{-1}\), respectively. How many moles of \(\mathrm{HCl}\) and \(\mathrm{H}_2 \mathrm{SO}_4\), respectively, will be needed to react completely with 1 mole of the metal hydroxide?
CorrectIncorrect -
Question 20 of 50
20. Question
1 point(s)The first ionization energy (in \(\mathrm{kJ} / \mathrm{mol}\) ) of \(\mathrm{Na}, \mathrm{Mg}, \mathrm{Al}\) and \(\mathrm{Si}\) respectively, are:
CorrectIncorrect -
Question 21 of 50
21. Question
1 point(s)The increasing order of the atomic radii of the following elements is:
(i) \(\mathrm{C}\)
(ii) \(\mathrm{O}\)
(iii) \(\mathrm{F}\)
(iv) \(\mathrm{Cl}\)
(v) \(\mathrm{Br}\)CorrectIncorrect -
Question 22 of 50
22. Question
1 point(s)The electron gain enthalpy (in \(\mathrm{kJ} / \mathrm{mol}\) ) of fluorine, chlorine, bromine and iodine, respectively, are:
CorrectIncorrect -
Question 23 of 50
23. Question
1 point(s)Within each pair of elements \(\mathrm{F~} \& \mathrm{~Cl}, \mathrm{S~} \& \mathrm{~Se}\), and \(\mathrm{Li~} \& \mathrm{~Na}\), respectively, the elements that release more energy upon an electron gain are:
CorrectIncorrect -
Question 24 of 50
24. Question
1 point(s)In comparison to boron, berylium has:
CorrectIncorrect -
Question 25 of 50
25. Question
1 point(s)The correct order of the atomic radii of \(\mathrm{C}, \mathrm{Cs}, \mathrm{Al}\), and \(\mathrm{S}\) is :
CorrectIncorrect -
Question 26 of 50
26. Question
1 point(s)The correct option with respect to the Pauling electronegativity values of the elements is:
CorrectIncorrect -
Question 27 of 50
27. Question
1 point(s)The \(71^{\text {st }}\) electron of an element \(\mathrm{X}\) with an atomic number of 71 enters into the orbital:
CorrectIncorrect -
Question 28 of 50
28. Question
1 point(s)In general, the properties that decrease and increase down a group in the periodic table, respectively, are:
CorrectIncorrect -
Question 29 of 50
29. Question
1 point(s)The correct order of electron affinity is:
CorrectIncorrect -
Question 30 of 50
30. Question
1 point(s)Both lithium and magnesium display several similar properties due to the diagonal relationship; however, the one which is incorrect is :
CorrectIncorrect -
Question 31 of 50
31. Question
1 point(s)The group having isoelectronic species is:
CorrectIncorrect -
Question 32 of 50
32. Question
1 point(s)Consider the following ionization enthalpies of two elements ‘A’ and ‘B’.
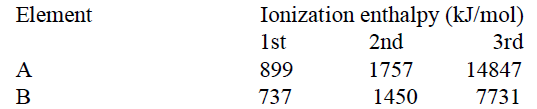
Which of the following statements is correct?
CorrectIncorrect -
Question 33 of 50
33. Question
1 point(s)Which of the following atoms has the highest first ionization energy?
CorrectIncorrect -
Question 34 of 50
34. Question
1 point(s)The following statements concern elements in the periodic table. Which of the following is true?
CorrectIncorrect -
Question 35 of 50
35. Question
1 point(s)The ionic radii (in Å) of \(\mathrm{N}^{3-}, \mathrm{O}^{2-}\) and \(\mathrm{F}^{-}\)are respectively:
CorrectIncorrect -
Question 36 of 50
36. Question
1 point(s)In the long form of the periodic table, the valence shell electronic configuration of \(5 s^2 5 p^4\) corresponds to the element present in :
CorrectIncorrect -
Question 37 of 50
37. Question
1 point(s)Which of the following series correctly represents relations between the elements from \(\mathrm{X}\) to \(\mathrm{Y}\) ?
\(\mathrm{X} \rightarrow \mathrm{Y}\)CorrectIncorrect -
Question 38 of 50
38. Question
1 point(s)Which of the following arrangements represents the increasing order (smallest to largest) of ionic radii of the given species \(\mathrm{O}^{2-}, \mathrm{S}^{2-}, \mathrm{N}^{3-}, \mathrm{P}^{3-}\)?
CorrectIncorrect -
Question 39 of 50
39. Question
1 point(s)Which of the following represents the correct order of increasing first ionization enthalpy for \(\mathrm{Ca}, \mathrm{Ba}, \mathrm{S}, \mathrm{Se}\) and \(\mathrm{Ar}\) ?
CorrectIncorrect -
Question 40 of 50
40. Question
1 point(s)The order of increasing sizes of atomic radii among the elements \(\mathrm{O}\), \(\mathrm{S}\), Se and As is :
CorrectIncorrect -
Question 41 of 50
41. Question
1 point(s)Identify the correct order of acidic strengths of \(\mathrm{CO}_2, \mathrm{CuO}, \mathrm{CaO}, \mathrm{H}_2 \mathrm{O}\)
CorrectIncorrect -
Question 42 of 50
42. Question
1 point(s)The correct order of radii is
CorrectIncorrect -
Question 43 of 50
43. Question
1 point(s)The correct order of acidic strength is
CorrectIncorrect -
Question 44 of 50
44. Question
1 point(s)Amongst \(\mathrm{H}_2 \mathrm{O}, \mathrm{H}_2 \mathrm{~S}, \mathrm{H}_2 \mathrm{Se}\) and \(\mathrm{H}_2 \mathrm{Te}\), the one with the highest boiling point is
CorrectIncorrect -
Question 45 of 50
45. Question
1 point(s)Which has most stable +2 oxidation state :
CorrectIncorrect -
Question 46 of 50
46. Question
1 point(s)Amongst the following elements (whose electronic configurations are given below), the one having the highest ionization energy is :
CorrectIncorrect -
Question 47 of 50
47. Question
1 point(s)Which one of the following is the strongest base?
CorrectIncorrect -
Question 48 of 50
48. Question
1 point(s)Which one of the following is the smallest in size?
CorrectIncorrect -
Question 49 of 50
49. Question
1 point(s)The first ionisation potential of \(\mathrm{Na}, \mathrm{Mg}, \mathrm{Al}\) and \(\mathrm{Si}\) are in the order
CorrectIncorrect -
Question 50 of 50
50. Question
1 point(s)The electronegativity of the following elements increases in the order
CorrectIncorrect