Chemical Bonding and Molecular Structure Quiz-1
Multiple Choice questions with one correct Answer
Quiz Summary
0 of 50 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 50 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 50
1. Question
1 point(s)The dipole moments of \(\mathrm{CCl}_4, \mathrm{CHCl}_3\) and \(\mathrm{CH}_4\) are in the order:
CorrectIncorrectHint
\(\mathrm{CHCl}_3\) is the only polar compound given among the two.
\(\mathrm{CH}_4=\mathrm{CCl}_4<\mathrm{CHCl}_3\). In other words we can also say that methane and carbon tetrachloride have zero dipole moment. -
Question 2 of 50
2. Question
1 point(s)Which of the following compounds contain(s) no covalent bond(s)? \(\mathrm{KCl}, \mathrm{PH}_3, \mathrm{O}_2, \mathrm{~B}_2 \mathrm{H}_6, \mathrm{H}_2 \mathrm{SO}_4\)
CorrectIncorrectHint
A covalent bond is formed between two nonmetals and an Ionic bond is formed between one metal and one nonmetal.
So, in \(\mathrm{KCl}, \mathrm{K}\) is metal and \(\mathrm{Cl}\) is nonmetal. Hence, It is an ionic bond. \(\mathrm{B}_2 \mathrm{H}_6, \mathrm{PH}_3\) and \(\mathrm{H}_2 \mathrm{SO}_4\) contains covalent bonds.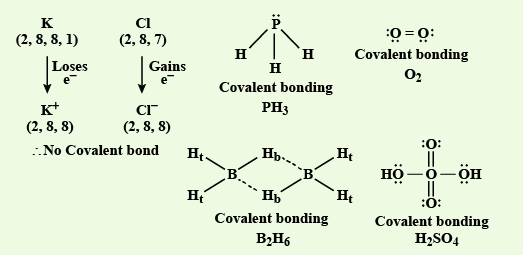
-
Question 3 of 50
3. Question
1 point(s)Which compound exhibits the maximum dipole moment among the following?
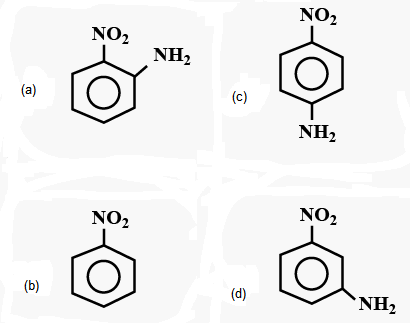 CorrectIncorrect
CorrectIncorrectHint
Dipole moment \(=(\) Distance between opposite charges \() \times(\) charge,\(q)\) \(\mu=q \times d\)
So, the greater the distance between the opposite charges higher the dipole. Due to the resonance, the greater charge separation occurs between charges due to linearity.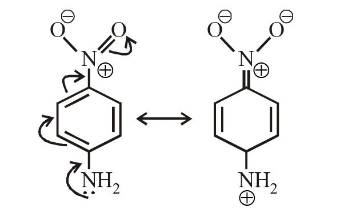
-
Question 4 of 50
4. Question
1 point(s)Which of these statements is not true?
CorrectIncorrectHint
(c)
\(
\begin{aligned}
& \mathrm{NO}^{+}=7+8-1=14 \mathrm{e}^{-} . \\
& \mathrm{O}_2=16 \mathrm{e}^{-} \text {i.e., not isoelectronic. }
\end{aligned}
\)
Boron forms only covalent compounds. This is due to its extremely high ionisation energy. Compounds of \(\mathrm{Tl}^{+}\)are much more stable than those of \(\mathrm{Tl}^{3+}\).
\(\mathrm{LiAlH}_4\) is a versatile reducing agent in organic synthesis. -
Question 5 of 50
5. Question
1 point(s)The correct order of bond dissociation energy among \(\mathrm{N}_2, \mathrm{O}_2, \mathrm{O}_2^{-}\)is shown in which of the following arrangements?
CorrectIncorrectHint
(c) The bond order of \(\mathrm{N}_2, \mathrm{O}_2\), and \(\mathrm{O}_2^{-}\)are 3,2 and 1.5 respectively. Since higher bond order implies higher bond dissociation energy, hence the correct order will be
\(
\mathrm{N}_2>\mathrm{O}_2>\mathrm{O}_2^{-}
\) -
Question 6 of 50
6. Question
1 point(s)Which one of the following molecules is polar?
CorrectIncorrectHint
(b) The geometry of \(\mathrm{IF}_5\) is square pyramide with an unsymmetric charge distribution, therefore this molecule is polar.
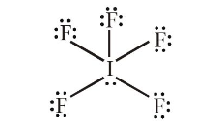
-
Question 7 of 50
7. Question
1 point(s)The geometry of \(\mathrm{H}_2 \mathrm{~S}\) and its dipole moment are
CorrectIncorrectHint
(a) Hybridisation of \(\mathrm{S}\) in \(\mathrm{H}_2 \mathrm{~S}=\frac{1}{2}(6+2+0-0)=4\)
\(\therefore \mathrm{S}\) has \(s p^3\) hybridisation and 2 lone pair of electrons in \(\mathrm{H}_2 \mathrm{~S}\).
\(\therefore\) It has angular geometry and so it has non-zero value of dipole moment. -
Question 8 of 50
8. Question
1 point(s)The critical temperature of water is higher than that of \(\mathrm{O}_2\) because the \(\mathrm{H}_2 \mathrm{O}\) molecule has
CorrectIncorrectHint
The critical temperature is directly proportional to the intermolecular force of attraction. \(\mathrm{H}_2 \mathrm{O}\) is a polar molecule, has greater intermolecular force of attraction than \(\mathrm{O}_2\), hence higher critical temperature.
The critical temperature of the water is higher than \(\mathrm{O}_2\) because \(\mathrm{H}_2 \mathrm{O}\) molecule has dipole moment which is due to its V-shape. -
Question 9 of 50
9. Question
1 point(s)Which contains both polar and non-polar bonds?
CorrectIncorrectHint
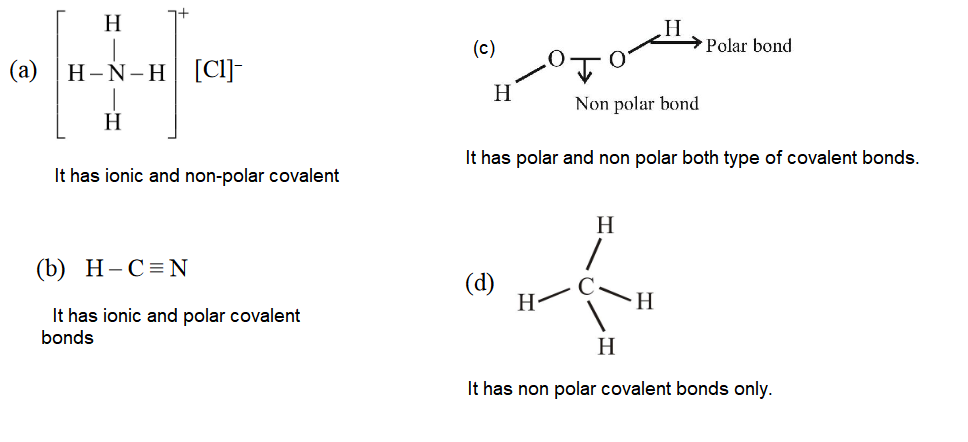
-
Question 10 of 50
10. Question
1 point(s)Which one is most ionic:
CorrectIncorrectHint
(i) Non-metallic oxides are more covalent (or less ionic) as compared to metallic oxides.
(ii) Higher the polarising power of cation (higher for higher oxidation state of similar size cations) more will be covalent character.
(a) \(\mathrm{P}_2 \mathrm{O}_5\) will be more covalent than other metallic oxides.
(b) Oxidation state of \(\mathrm{Mn}\) is +7 in \(\mathrm{Mn}_2 \mathrm{O}_7\), oxidation state of \(\mathrm{Cr}\) in \(\mathrm{CrO}_3\) is +6 and oxidation state of \(\mathrm{Mn}\) is +2 in \(\mathrm{MnO}\).
\(\therefore \mathrm{MnO}\) is most ionic.NOTE : \(\mathrm{P}_2 \mathrm{O}_5\), being a non-metallic oxide will definitely be more covalent than the other metallic oxides. Further, we know that higher the polarising power of the cation (higher for higher oxidation state of the similar size cations) more will be the covalent character. Here \(\mathrm{Mn}\) is in +7 O.S in \(\mathrm{Mn}_2 \mathrm{O}_7\), Cr in +6 in \(\mathrm{CrO}_3\) and \(\mathrm{Mn}\) in +2 in \(\mathrm{MnO}\). So, \(\mathrm{MnO}\) is the most ionic and \(\mathrm{Mn}_2 \mathrm{O}_7\) is the most covalent.
-
Question 11 of 50
11. Question
1 point(s)Pick out the isoelectronic structures from the following;
I. \(\mathrm{CH}_3^{+}\)
II. \(\mathrm{H}_3 \mathrm{O}^{+}\)
III. \(\mathrm{NH}_3\)
IV \(\mathrm{CH}_3^{-}\)CorrectIncorrectHint
(d) No. of \(\mathrm{e}^{-}\)in \(\mathrm{CH}_3^{+}=6+3-1=8\)
No. of \(\mathrm{e}^{-}\)in \(\mathrm{H}_3 \mathrm{O}^{+}=3+8-1=10\)
No. of \(\mathrm{e}^{-}\)in \(\mathrm{NH}_3=7+3=10\)
No. of \(\mathrm{e}^{-}\)in \(\mathrm{CH}_3^{-}=6+3+1=10\)
\(\therefore \mathrm{H}_3 \mathrm{O}^{+}, \mathrm{NH}_3\) and \(\mathrm{CH}_3^{-}\)are isoelectronic. -
Question 12 of 50
12. Question
1 point(s)The cyanide ion, \(\mathrm{CN}^{-}\)and \(\mathrm{N}_2\) are isoelectronic. But in contrast to \(\mathrm{CN}^{-}\), \(\mathrm{N}_2\) is chemically inert, because of
CorrectIncorrectHint
(b) In \(\mathrm{N}_2\), similar atoms are linked to each other and thus there is no polarity.
The cyanide ions, \(\mathrm{CN}^{-}\)and \(\mathrm{N}_2\) are isoelectronic, but in contrast to \(\mathrm{CN}^{-}, \mathrm{N}_2\) is chemically inert because of the absence of polarity of bond. Cyanide ion is negatively charged but \(\mathrm{N}_2\) is a neutral molecule.
-
Question 13 of 50
13. Question
1 point(s)The molecule which has zero dipole moment is :
CorrectIncorrectHint
(b) The dipole moment of compound having regular geometry and same type of atoms is zero. It is vector quantity. The zero dipole moment of \(\mathrm{BF}_3\) is due to its symmetrical (triangular planar) structure. The three fluorine atoms lie at the corners of an equilateral triangle with boron at the centre.
NOTE: The vectorial addition of the dipole moments of the three bonds gives a net sum of zero because the resultant of any two dipole moments is equal and opposite to the third. The dipole moment of \(\mathrm{NH}_3\) is \(1.46 \mathrm{D}\) indicating its unsymmetrical structure. The dipole moment of \(\mathrm{CH}_2 \mathrm{Cl}_2\) (the molecule uses \(s p^3\) hybridisation but is not symmetric) is \(1.57 \mathrm{D}\).
-
Question 14 of 50
14. Question
1 point(s)The bond between two identical non-metal atoms has a pair of electrons:
CorrectIncorrectHint
(d) In covalent bonds, between two identical non-metal, atoms share the pair of electrons equally between them, e.g. : \(\mathrm{F}_2, \mathrm{O}_2, \mathrm{~N}_2\).
-
Question 15 of 50
15. Question
1 point(s)The types of bonds present in \(\mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}\) are only
CorrectIncorrectHint
(c) Ionic bond or electrovalent between \(\mathrm{Cu}^{2+}\) and \(\mathrm{SO}_4^{2-}\), covalent and coordinate in \(\mathrm{SO}_4^{2-}\);
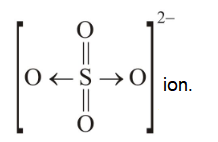
-
Question 16 of 50
16. Question
1 point(s)Carbon tetrachloride has no net dipole moment because of
CorrectIncorrectHint
(b) In regular tetrahedral structure, dipole moment of one bond is cancelled by opposite dipole moment of the other bonds.
-
Question 17 of 50
17. Question
1 point(s)The compound with no dipole moment is
CorrectIncorrectHint
(i) Dipole moment is vector quantity. When vector sum of all dipoles in molecule will be zero, then molecule will not have net dipole moment.
(ii) NOTE : For net dipole moment to be equal to zero, all the atoms attached to central atom must be identical and geometry must be regular.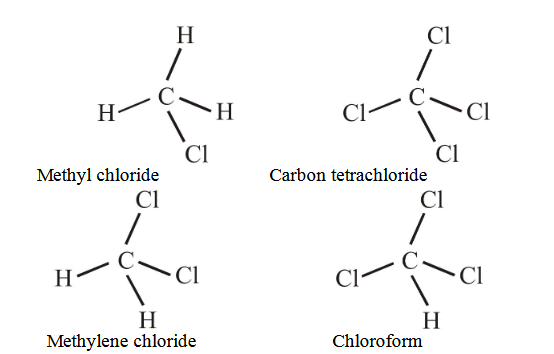
Therefore, Carbon tetrachloride having regular geometry and identical atoms attached to bonds has zero dipole moment.
-
Question 18 of 50
18. Question
1 point(s)The ion that is isoelectronic with \(\mathrm{CO}\) is
CorrectIncorrectHint
(a) NOTE : Isoelectronic species have same number of electrons. Electrons in \(\mathrm{CO}=6+8=14\)
Electrons in \(\mathrm{CN}^{-}=6+7+1=14\)
Electrons in \(\mathrm{O}_2^{-}=8+8+1=17\)
Electrons in \(\mathrm{O}_2^{+}=8+8-1=15\)
\(\therefore \mathrm{CO}\) and \(\mathrm{CN}^{-}\)are isoelectronic. -
Question 19 of 50
19. Question
1 point(s)If a molecule \(M X_3\) has zero dipole moment, the sigma bonding orbitals used by \(M\) (atomic number \(<21\) ) are
CorrectIncorrectHint
(c) NOTE: Dipole moment is vector quantity.
In trigonal planar geometry (for \(s p^2\) hybridisation), the vector sum of two bond moments is equal and opposite to the dipole moment of the third bond.
-
Question 20 of 50
20. Question
1 point(s)Which of the following is soluble in water
CorrectIncorrectHint
(b) It forms hydrogen bonds with water
Explanation for the correct option:
b. \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}\)
– Water is a polar solvent with hydrogen bonding, ethyl alcohol molecules should have polarity or \(\mathrm{H}\)-bond in order to dissolve in water.
– Ethyl alcohol possesses hydrogen bonding as well as being polar due to the strong electronegativity of oxygen; as a result, it is water-soluble.Explanation for incorrect options:
a. \(\mathrm{CS}_2\)
– \(\mathrm{CS}_2\) is non-polar.
– It won’t dissolve in water.
c. \(\mathrm{CCl}_4\)
– \(\mathrm{CCl}_4\) is non-polar in the third scenario. Any bond dipoles \((\mathrm{C}-\mathrm{Cl})\) will cancel out because of the molecule’s tetrahedral symmetry.
– It will be water-insoluble.
d. \(\mathrm{CHCl}_3\)
– Chloroform is a moderately polar chemical.
– However, it is unable to form any strong bonds (such as hydrogen bonds) with water.
– Its solubility in water is quite low due to the lack of a strong contact with water. -
Question 21 of 50
21. Question
1 point(s)The total number of electrons that take part in forming the bond in \(\mathrm{N}_2\) is
CorrectIncorrectHint
(c)

\(\mathrm{N}_2\) has triple bond and each covalent bond is associated with one pair of electrons, therefore, six electrons are involved in forming bonds in \(\mathrm{N}_2\)
-
Question 22 of 50
22. Question
1 point(s)Which of the following compounds are covalent?
CorrectIncorrectHint
Hydrogen \(\left(\mathrm{H}_2\right)\) is a covalent molecule as it is formed by sharing a pair of electron between two atoms of same element. \(\mathrm{CaO}, \mathrm{KCl}\) and \(\mathrm{Na}_2 \mathrm{~S}\) are ionic as they are formed between atoms of elctropositive and electronegative elements.
-
Question 23 of 50
23. Question
1 point(s)Element \(X\) is strongly electropositive and element \(Y\) is strongly electronegative. Both are univalent. The compound formed would be
CorrectIncorrectHint
(a) \(X^{+} Y^{-}\)
\(\because\) Electropositive elements forms cation and electronegative elements forms anion. Both are held together by electrostatic forces of attraction. -
Question 24 of 50
24. Question
1 point(s)The octet rule is not valid for the molecule
CorrectIncorrectHint
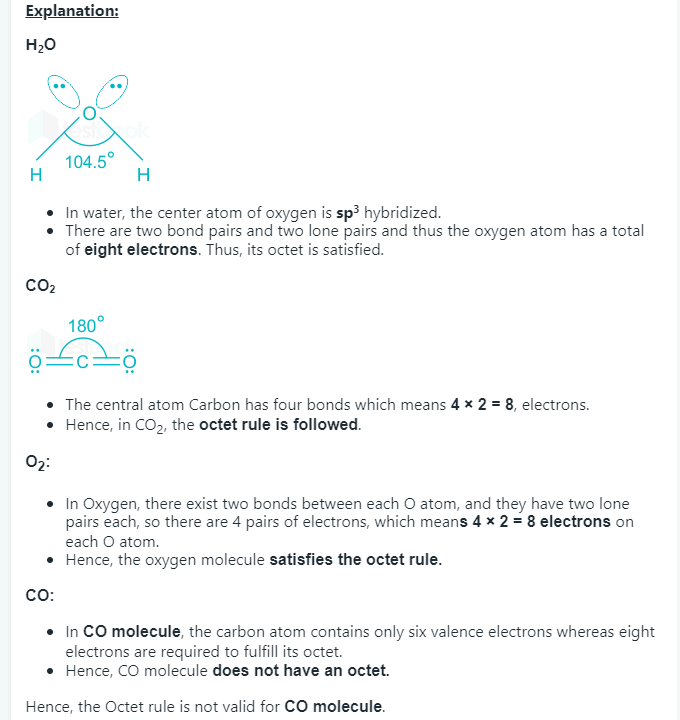
-
Question 25 of 50
25. Question
1 point(s)The compound which contains both ionic and covalent bonds is
CorrectIncorrectHint
(c) In \(\mathrm{KCN}\), ionic bond is present between \(\mathrm{K}^{+}\)and \(\mathrm{CN}^{-}\)and covalent bonds are present between carbon and nitrogen \(\mathrm{C} \equiv \mathrm{N}\).
-
Question 26 of 50
26. Question
1 point(s)The two types of bonds present in \(\mathrm{B}_2 \mathrm{H}_6\) are covalent and ____.
CorrectIncorrectHint
Three centred two-electron bonds or banana bond;
NOTE : The formation of three centred two-electron bond is due to one empty \(s p^3\) orbital of one of the B atom, \(1 s\) orbital of the bridge hydrogen atom and one of the \(s p^3\) (filled) orbital of the other B-atom. This forms a delocalized orbital covering the three nuclei giving the shape of a banana. Thus, also known as banana bonds.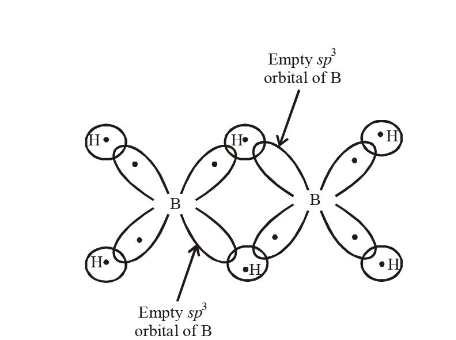
-
Question 27 of 50
27. Question
1 point(s)There are ______ \(\pi\) bonds in a nitrogen molecule.
CorrectIncorrectHint
\(\mathrm{N} \equiv \mathrm{N}\left(\mathrm{N}_2\right)\) has \(1 \sigma\) and \(2 \pi\) bonds. (A triple bond consists of \(1 \sigma\) and \(2 \pi\) bonds)
-
Question 28 of 50
28. Question
1 point(s)The compound that has the largest \(\mathrm{H}-\mathrm{M}-\mathrm{H}\) bond angle \((\mathrm{M}=\mathrm{N}, \mathrm{S}\), C), is:
CorrectIncorrectHint
\(\mathrm{CH}_4\) has the largest \(\mathrm{H}-\mathrm{M}-\mathrm{H}\) bond angle which is \(109.5^{\circ}\).
The bond angle of other species are given below:
\(\mathrm{H}_2 \mathrm{O}-104.5^{\circ}\left(s p^3\right.\) with 2 lone pair at \(\left.\mathrm{O}\right)\)
\(\mathrm{NH}_3-107^{\circ}\left(s p^3\right.\) with 1 lone pair at \(\left.\mathrm{N}\right)\)
\(
\mathrm{CH}_4-109.5^{\circ}\left(s p^3\right)
\)
\(\mathrm{H}_2 \mathrm{~S}-92^{\circ}\left(s p^3\right.\) with 2 lone pair at \(\left.\mathrm{O}\right)\)
Lone pair-bond pair repulsion in \(\mathrm{H}_2 \mathrm{~S}\) will increase because ‘ \(\mathrm{S}\) ‘ has lower electronegativity than ‘O’. So there will be lesser electron density on ‘S’ and thus \(\mathrm{H}-\mathrm{S}-\mathrm{H}\) bond angle will be smaller than \(\mathrm{H}_2 \mathrm{O}\). -
Question 29 of 50
29. Question
1 point(s)The molecule in which hybrid Molecular Orbitals involve only one \(d\)-orbital of the central atom is :
CorrectIncorrectHint
(a) \(\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}=d s p^2\)
(b) \(\mathrm{BrF}_5=s p^3 d^2\)
(c) \(\mathrm{XeF}_4=s p^3 d^2\)
(d) \(\left[\mathrm{CrF}_6\right]^{3-}=d^2 s p^2\)
The molecule in which hybrid MOs involve only one d-orbital of the central atom is \(\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}\) as in this complex \(\mathrm{Ni}^{2+}\) undergoes \(dsp^2\) hybridization -
Question 30 of 50
30. Question
1 point(s)If \(\mathrm{AB}_4\) molecule is a polar molecule, a possible geometry of \(\mathrm{AB}_4\) is :
CorrectIncorrectHint
For \(\mathrm{AB}_4\) compound possible geometry are
\(
\begin{array}{ccc}
\text { No. of Bond pair } & \text { No. of lone pair } & \text { Hybridisation } \\
4 & 0 & s p^3 \\
4 & 1 & s p^3 d \\
4 & 2 & s p^3 d^2
\end{array}
\)
Structure with \(s p^3 d^2\) hybridisation is polar due to lone pair moment while in other possibilities molecules is non-polar.
Square pyramidal can be polar due to lone pair moment as the bond pair moments will get cancelled out. -
Question 31 of 50
31. Question
1 point(s)The shape/ structure of \(\left[\mathrm{XeF}_5\right]^{-}\)and \(\mathrm{XeO}_3 \mathrm{F}_2\), respectively, are :
CorrectIncorrectHint
(i) \(\mathrm{XeF}_5^{-} \quad\) St. No. = Bond pair + Lone Pair
\(
=(5+2)=7
\)So, hybridisation is \(=s p^3 d^\beta\)
and the structure is pentagonal planar.
\(\begin{array}{ll}\text { (ii) } \mathrm{XeO}_3 \mathrm{~F}_2 & \text { St. No. }=5\end{array}\)
So, hybridisation is \(=s p^3 d\) and structure is trigonal bipyramidal. -
Question 32 of 50
32. Question
1 point(s)The molecular geometry of \(\mathrm{SF}_6\) is octahedral. What is the geometry of \(\mathrm{SF}_4\) (including lone pair(s) of electrons, if any)?
CorrectIncorrectHint
(b) \(\mathrm{SF}_4\)
Bond pair \(=4\)
Lone pair \(=1\)
Steric number \(=5\),
So, hybridisation is \(s p^3 d\).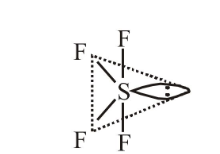
Geometry is trigonal bipyramidal but the shape is “See Saw”.
-
Question 33 of 50
33. Question
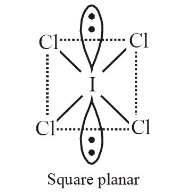
1 point(s)The correct statement about \(\mathrm{ICl}_5\) and \(\mathrm{ICl}_4^{-}\)is
CorrectIncorrectHint
(d) \(\mathrm{ICl}_5\) is \(s p^3 d^2\) hybridised ( \(\left.5 b p, 1 l p\right)\)
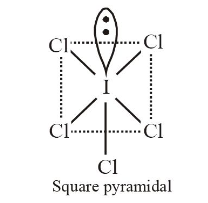
\(\mathrm{ICl}_4^{-}\)is \(s p^3 d^2\) hybridised \((4 b p, 2 l p)\)
-
Question 34 of 50
34. Question
1 point(s)The ion that has \(s p^3 d^2\) hydridisation for the central atom, is:
CorrectIncorrectHint
\(
\begin{array}{ll}
\text { (a) Species } & \text { Hybridisation } \\
\mathrm{ICl}_2^{-} & s p^3 d \\
\mathrm{ICl}_4^{-} & s p^3 d^2 \\
\mathrm{BrF}_2^{-} & s p^3 d \\
\mathrm{IF}_6^{-} & s p^3 d^3
\end{array}
\) -
Question 35 of 50
35. Question
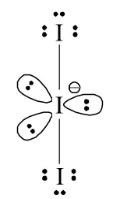
1 point(s)Total number of lone pair of electrons in \(\mathrm{I}_3^{-}\)ion is :
CorrectIncorrectHint
Total number of lone pair of electrons is 9.
-
Question 36 of 50
36. Question
1 point(s)The incorrect geometry is represented by
CorrectIncorrectHint
(a) \(\mathrm{NF}_3\) has trigonal pyramidal geometry. \(\mathrm{N}\) atom has one lone pair and three bond pairs of electrons. The electron pair geometry is tetrahedral and molecular geometry is trigonal pyramidal. The bond angles are lower than tetrahedral bond angles due to lone pair – lone pair and lone pair – bond pair repulsions. \(\mathrm{N}\) atom is \(s p^3\) hybridised.
-
Question 37 of 50
37. Question
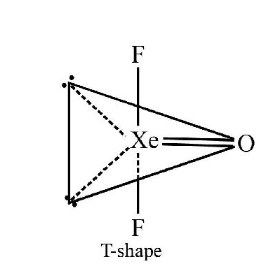
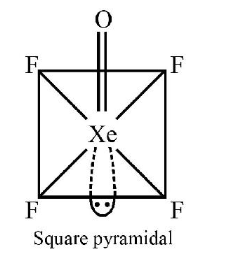
1 point(s)Identify the pair in which the geometry of the species is T-shape and square pyramidal, respectively
CorrectIncorrectHint
\(
\mathrm{XeOF}_2
\)
\(
\mathrm{XeOF}_4
\)
-
Question 38 of 50
38. Question
1 point(s)\(s p^3 d^2\) Hybridisation is not displayed by :
CorrectIncorrectHint
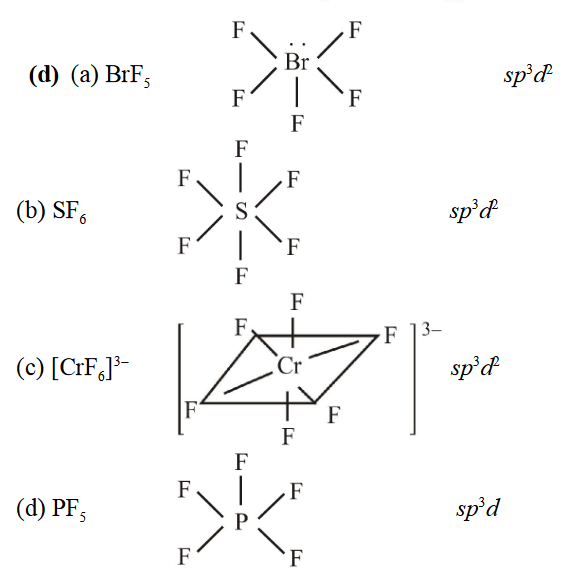
-
Question 39 of 50
39. Question
1 point(s)The species in which the \(\mathrm{N}\) atom is in a state of \(s p\) hybridisation is
CorrectIncorrectHint
(c) Hybridisation \((\mathrm{H})=[\) No. of valence electrons of central atom + No. of monovalent atoms attached to it + (-ve charge if any) \(-(+\mathrm{ve}\) charge if any)]
\(
\mathrm{NO}_2^{+}=\text {i.e. } s p \text { hybridisation }
\)
\(
\mathrm{NO}_2^{-}=\text {i.e. } s p^2 \text { hybridisation }
\)
\(
\mathrm{NO}_3^{-}=\text {i.e. } s p^2 \text { hybridisation }
\)
The Lewis structure of \(\mathrm{NO}_2\) shows a bent molecular geometry with trigonal planar electron pair geometry hence the hybridization will be \(s p^2\). -
Question 40 of 50
40. Question
1 point(s)The group of molecules having identical shape is:
CorrectIncorrectHint
(d) \(\mathrm{ClF}_3 \longrightarrow\) Hybridisation \(=3+\frac{1}{2}[7-3]=5\left(s p^3 d\right)\)
\(
\begin{aligned}
& \mathrm{XeOF}_2 \longrightarrow \text { Hybridisation }=3+\frac{1}{2}[8-4]=5\left(s p^3 d\right) \\
& \mathrm{XeF}_3^{+} \longrightarrow \text { Hybridisation }=3+\frac{1}{2}[8-3-1]=5\left(s p^3 d\right)
\end{aligned}
\)All molecules have \(s p^3 d\) hybridisation and 2 lone pairs. Hence all have identical (T-shape).
-
Question 41 of 50
41. Question
1 point(s)The number and type of bonds in \(\mathrm{C}_2^{2-}\) ion in \(\mathrm{CaC}_2\) are:
CorrectIncorrectHint
\(
\mathrm{C}_2^{2-} \text { ion } \rightarrow[\mathrm{C} \equiv \mathrm{C}]^{2-}
\)
Dicarbide ion \(\mathrm{C}_2^{2-}\) has 1 sigma and 2 \(\pi\) bonds. There are two \(\pi\) bonds and one \(\sigma\) bond in a triple bond. -
Question 42 of 50
42. Question
1 point(s)The shape of \(\mathrm{IF}_6^{-}\)is:
CorrectIncorrectHint
The shape of \(\mathrm{IF}_6^{-}\)is trigonally distorted octahedron. The central iodine atom has 7 valence electrons. It gains one electron from negative charge. Out of 8 valence electrons, 6 are involved in formation of \(6 \mathrm{I}-\mathrm{F}\) bonds and one lone pair of electrons is present. \(6 \mathrm{~F}\) atoms are present at 6 corners of distorted octahedron. The lone pair of electrons distorts the octahedral shape due to electron-electron repulsion. This is due to the presence of a “weak” lone pair.
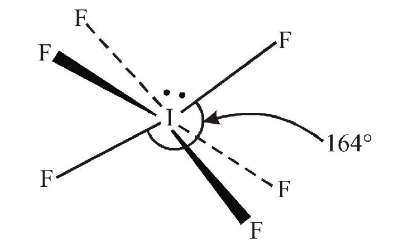
-
Question 43 of 50
43. Question
1 point(s)The species having pyramidal shape is :
CorrectIncorrectHint
(d) \(\mathrm{OSF}_2: H=\frac{6+2}{2}=4\). It has 1 lone pair.
\(
\text { The shapes of } \mathrm{SO}_3, \mathrm{BrF}_3 \text { and } \mathrm{SiO}_3^{2-} \text { are triangular planar respectively. }
\)
-
Question 44 of 50
44. Question
1 point(s)Which species has the maximum number of lone pair of electrons on the central atom?
CorrectIncorrectHint
\(\mathrm{I}_3^{-}\)has the maximum number of lone pair of electron on the central atom. \(\mathrm{I}_3^{-}, \mathrm{XeF}_4, \mathrm{SF}_4\) and \(\mathrm{ClO}_3^{-}\)have \(3,2,1,1\) lone pair of electron respectively.
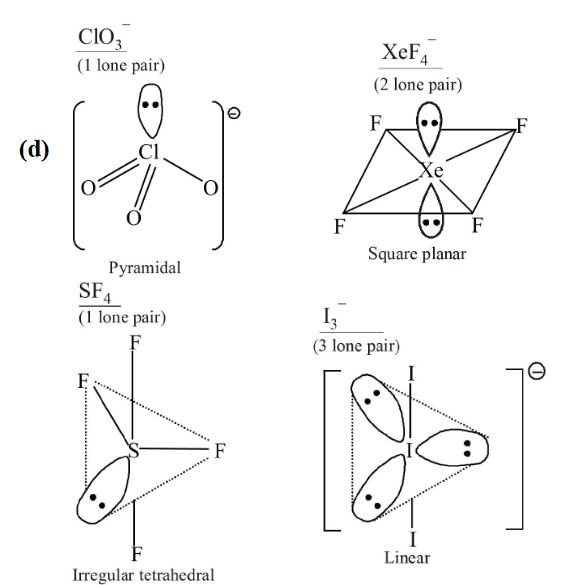
-
Question 45 of 50
45. Question
1 point(s)Which of the following are isoelectronic and isostructural? \(\mathrm{NO}_3^{-}\), \(\mathrm{CO}_3^{2-}, \mathrm{ClO}_3^{-}, \mathrm{SO}_3\)
CorrectIncorrectHint
(a) NOTE : Isoelectronic species have same number of electrons and isostructural species have same type of hybridisation at central atom.
\(
\mathrm{NO}_3^{-} \text {; No. of } \mathrm{e}^{-}=7+8 \times 3+1=32 \text {, hybridisation of } \mathrm{N} \text { in } \mathrm{NO}_3^{-} \text {is } s p^3
\)
\(
\mathrm{CO}_3^{2-} ; \text { No. of } \mathrm{e}^{-}=6+8 \times 3+2=32 \text {, hybridisation of } \mathrm{C} \text { in } \mathrm{CO}_3^{2-} \text { is } s p^3
\)
\(
\mathrm{ClO}_3^{-} \text {; No. of } \mathrm{e}^{-}=17+8 \times 3+1=42 \text {, hybridisation of } \mathrm{Cl} \text { in } \mathrm{ClO}_3^{-} \text {is } s p^3
\)
\(\mathrm{SO}_3 ;\) No. of \(\mathrm{e}^{-}=16+8 \times 3=40\), hybridisation of \(\mathrm{S}\) in \(\mathrm{SO}_3\) is \(s p^2\) \(\therefore \mathrm{NO}_3^{-}\)and \(\mathrm{CO}_3^{2-}\) are isostructural and isoelectronic. -
Question 46 of 50
46. Question
1 point(s)Specify the coordination geometry around and hybridisation of \(\mathrm{N}\) and B atoms in a 1:1 complex of \(\mathrm{BF}_3\) and \(\mathrm{NH}_3\)
CorrectIncorrectHint
\(
\text { (a) } \mathrm{H}_3 \mathrm{N} \rightarrow \mathrm{BF}_3 \text { where both } \mathrm{N} \text {, } \mathrm{B} \text { are attaining tetrahedral geomerty. }
\) -
Question 47 of 50
47. Question
1 point(s)The correct order of hybridization of the central atom in the following species \(\mathrm{NH}_3,\left[\mathrm{PtCl}_4\right]^{2-}, \mathrm{PCl}_5\) and \(\mathrm{BCl}_3\) is
CorrectIncorrectHint
\(
\text { Formulae : } H=\frac{1}{2}[V+M-C+A]
\)
\(
\begin{gathered}
\text { Hybridisation of } \mathrm{N} \text { in } \mathrm{NH}_3 \\
=\frac{1}{2}[5+3-0+0]=4 \quad \therefore s p^3
\end{gathered}
\)Hybridisation of \(\mathrm{Pt}\) in \(\left[\mathrm{PtCl}_4\right]^{2-}\)
\(
=\frac{1}{2}[2+4-0+2]=4 \quad \therefore d s p^2
\)Hybridisation of \(\mathrm{P}\) in \(\mathrm{PCl}_5\)
\(
=\frac{1}{2}[5+5-0+0]=5 \quad \therefore s p^3 d
\)Hybridisation of \(\mathrm{B}\) in \(\mathrm{BCl}_3\)
\(
=\frac{1}{2}[3+3-0+0]=3 \quad \therefore s p^2
\) -
Question 48 of 50
48. Question
1 point(s)The hybridisation of atomic orbitals of nitrogen in \(\mathrm{NO}_2^{+}, \mathrm{NO}_3^{-}\)and \(\mathrm{NH}_4^{+}\)are
CorrectIncorrectHint
(b) For \(\mathrm{NO}_2^{+}: H=\frac{1}{2}(5+0+0-1)=2\);
\(\therefore s p\) hybridisationFor \(\mathrm{NO}_3^{-}: H=\frac{1}{2}[5+0+1-0)=3\);
\(\therefore s p^2\) hybridisation
For \(\mathrm{NH}_4^{+}: H=\frac{1}{2}[5+4+0-1]=4\);
\(\therefore s p^3\) hybridisation -
Question 49 of 50
49. Question
1 point(s)Molecular shapes of \(\mathrm{SF}_4, \mathrm{CF}_4\) and \(\mathrm{XeF}_4\) are
CorrectIncorrectHint
(d) The structure of species can be predicted on the basis of hybridisation which in turn can be known by knowing the number of hybrid orbitals \((H)\) in that species

\(
=\frac{1}{2}(6+4+0-0)=5
\)For \(\mathbf{S F}_4: \mathrm{S}\) is \(s p^3 d\) hybridised in \(\mathrm{SF}_4\). Thus \(\mathrm{SF}_4\) has 5 hybrid orbitals of which only four are used by \(\mathrm{F}\), leaving one lone pair of electrons on sulphur.
For \(\mathbf{C F}_4: H=\frac{1}{2}[4+4+0-0]=4 \therefore s p^3\) hybridisaion
Since all the four orbitals of carbon are involved in bond formation, no lone pair is present on \(\mathrm{C}\) having four valence electrons
For \(\mathrm{XeF}_4: H=\frac{1}{2}(8+4+0-0)=6, \therefore s p^3 d^2\) hybridization of the six hybrid orbitals, four form bond with \(\mathrm{F}\), leaving behind two lone pairs of electrons on Xe. -
Question 50 of 50
50. Question
1 point(s)The geometry and the type of hybrid orbital present about the central atom in \(\mathrm{BF}_3\) is
CorrectIncorrectHint
(b) \(\quad H=\frac{1}{2}(3+3+0-0)=3\)
\(\therefore\) Boron, in \(\mathrm{BF}_3\), is \(s p^2\) hybridised leading to trigonal planar shape.